Is condensing a physical change or chemical change?
In this process the water vapours present in steam transformed into a liquid, but there is no change in the composition of steam. The composition of steam will be the same in both liquid and vapours form. Hence the correct option is true i.e. the condensation of steam is not a chemical change it is a physical change.
Is condensing a gas a chemical change?
When chemical bonds are broken or formed, new particles are created. Therefore, frying an egg is a chemical change because it results in the formation of new particles. What is the change in condensation of steam is called? We know that condensation of a gas is actually is conversion of gas into its vapuors forms.
Is density a physical or a chemical?
is density a physical or chemical property ? Density depends upon the number of particles in a unit area. So it doesn’t matter what kind of particles are used. So it is a physical property.
Is mass and volume conserved in a chemical change?
So the volume is not conserved. However, the mass is conserved: the mass of the reactants is 28 + 16 = 44g, and the mass of the product is 44g. Assuming I understand the intent and context of the question, volume realistically comes into play when dealing with reactions involving gases.

Is density a chemical or physical change?
physical propertyDensity can be established simply by determining the mass and volume of substance, no reaction is involved, so its a physical property.
Is density example of chemical property?
The general properties of matter such as color, density, hardness, are examples of physical properties. Properties that describe how a substance changes into a completely different substance are called chemical properties. Flammability and corrosion/oxidation resistance are examples of chemical properties.
Is density evidence of a chemical change?
Volume Change Each chemical compound has a specific density. If the chemical compound changes due to a chemical reaction, the density changes as well. This causes the volume of the substance to recede or expand during the reaction process.
Is density a physical change?
To measure density, simply measure the mass on a balance, calculate volume from measured lengths and divide the two. This process involves no chemical change; therefore, density is a physical property.
Why is density considered a physical property?
The density of a substance remains constant and do not depend on the amount of substance. Also, the substance does not need to undergo any chemical reaction for identification of its density. Thus, density is considered to be as physical property.
Which of the following is not a chemical change?
The correct answer is Freezing of water. Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. Freezing of water is not a chemical change as ice when melt changes back to water showing the physical change.
Is density a chemical property of matter true or false?
Answer: FALSE Density is a physical property that relates the mass of a substance to its volume. We can observe and compare the density of...
Which is not true for a chemical change?
False - During a chemical change, the composition of the original substance is altered and the change is irreversible. False - Melting of butter and wax are examples of chemical changes. False - Melting of ice is an endothermic, reversible physical change.
What type of property is density?
intensive propertyDensity is an intensive property because there is a narrow range of densities across the samples. No matter what the initial mass was, densities were essentially the same. Since intensive properties do not depend on the amount of material, the data indicate that density is an intensive property of matter.
Which density is a physical property?
Density is a physical property of matter that expresses a relationship of mass to volume. The more mass an object contains in a given space, the more dense it is.
Is density a chemical property of matter true or false?
Answer: FALSE Density is a physical property that relates the mass of a substance to its volume. We can observe and compare the density of...
Which of the following is an example of a chemical property?
Examples of chemical properties include flammability, toxicity, acidity, reactivity (many types), and heat of combustion.
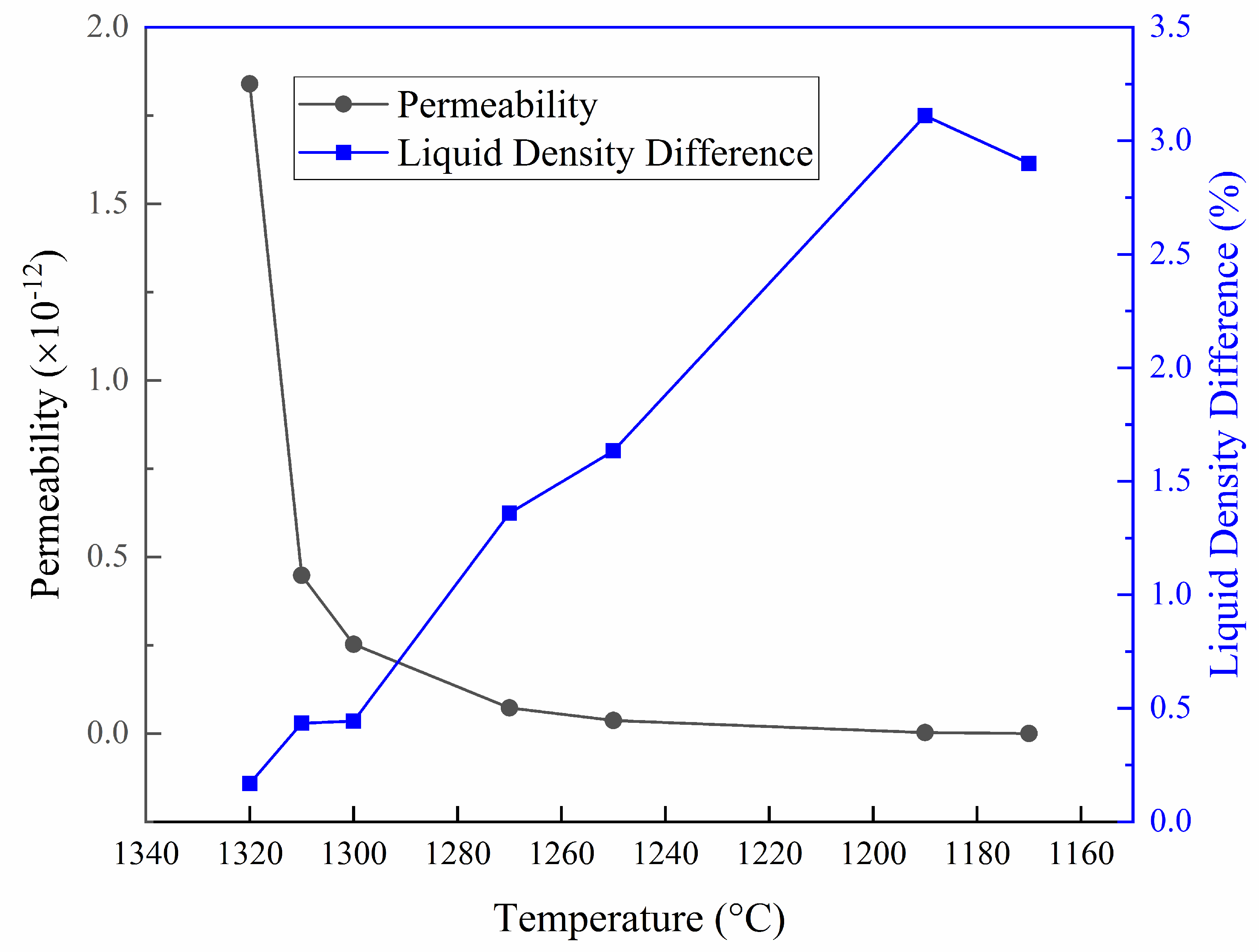
What happens to the density of a liquid at the beginning of melting?
At the beginning of melting, the density change by volume expansion generates an extra movement of liquid to accelerate melting. The effect of density change diminishes with time although the amount of excess melting fraction remains.
What is the change of densities of the phases B and C?
The change of densities of the phases B and C requires change of velocities u B, u C, which restores the mass balance, i.e., equality (3.15). But this modification of velocities breaks the momentum balance (3.16). This means that the modified motion of the phases implies some noncompensated forces.
What is volume change?
Volume change is a comparatively unspectacular rheological process, but occasionally a critical one. During melting and solidification density changes of up to 30 per cent may occur, while simple melts are readily compressed by 10 per cent under pressures of the order 10 8 N/m 2 (1000 atm).
How does density affect heat transfer?
Effect of density change In addition to the natural convection, solid-liquid density change influences on the phase change heat transfer. The significance in phase change process has been pointed out by some researchers in experimental studies. The first investigation to take account of this factor into the analysis was made by Shamsundar and Sparrow (1976), where only pure conduction was considered as the heat transport mechanism. It is shown that the volume-change-driven motion played a dominant role at early stage of melting and interacted with natural convective motion as melting proceeded ( Sparrow and Broadbent, 1982 ). Also, the omission of the density change effect in simulation resulted in disagreement between experimental and numerical data and its effect could not be neglected in the analysis ( Ho and Viskanta, 1984 ). To make a better prediction on the heat transfer characteristics during melting or solidification, it is obvious that not only natural convection in the melt but the density change should be included in the analysis.
Is melting rate proportional to velocity?
Melting rate at the base is proportional to the velocity of bulk motion. However, the effect of the velocity is not important in melting of other phase change faces. There may exist an optimum geometric aspect ratio with which maximum melting rate is obtained for a given amount of mass. FIGURE 5.
Stefan Problems
A.D. Solomon, in Encyclopedia of Physical Science and Technology (Third Edition), 2003
Compressible Flow
As density changes are accompanied by temperature changes, thermodynamic principles are constantly used throughout this chapter. Most of the necessary concepts and relations have been summarized in Sections 1.8 and 1.9, which may be reviewed before proceeding further.
Fundamentals for power engineering
Tomio Okawa, ... Daisuke Ito, in Fundamentals of Thermal and Nuclear Power Generation, 2021
Sustainability of Industrial Waste Management
Dr.Salah M. El-Haggar PE, PhD, in Sustainable Industrial Design and Waste Management, 2007
PROCESSING
Roy J. Crawford, James L. Throne, in Rotational Molding Technology, 2002
Testing Methods of Weathered Specimen
Weathered polymers usually undergo a density change.27,122,381–383 Density usually increases, but the direction it takes depends on the polymer ( Figure 13.42 ). It is apparent that density change is not an indicator of weather stability since PVC would be rated as more stable than PMMA, which is not the case.
Fundamentals of Fluid Simulation by the MPS Method
Seiichi Koshizuka, ... Takuya Matsunaga, in Moving Particle Semi-implicit Method, 2018