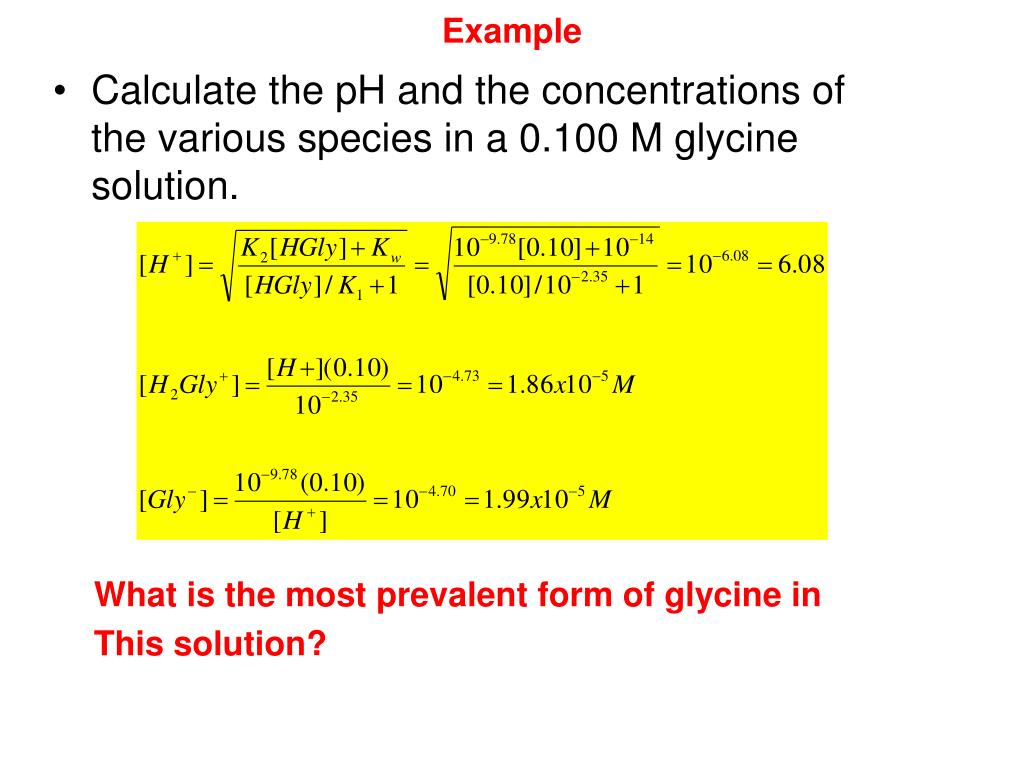
Glycine’s activity is fairly representative of that of the simpler amino acids. Since glycine is neither a strong acid nor a strong base, four species in rapid equilibrium are required to produce glycine in water.
Full Answer
Is glycine the same as glycerin?
Not to be confused with Glycerin. Glycine (symbol Gly or G; / ˈɡlaɪsiːn /) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH 2 ‐ CH 2 ‐ COOH. Glycine is one of the proteinogenic amino acids.
Is glycine a stable amino acid?
Glycine (symbol Gly or G; / ˈ ɡ l aɪ s iː n /) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH 2 ‐ CH 2 ‐ COOH .
What is the chemical formula of glycine?
It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH 2 ‐ CH 2 ‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form.
What is the standard state of glycine at 25 degrees Celsius?
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). ?) Glycine (symbol Gly or G; / ˈɡlaɪsiːn /) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH 2 ‐ CH 2 ‐ COOH.
What type of acid is glycine?
amino acidGlycine (symbol Gly or G; /ˈɡlaɪsiːn/) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids.
Can glycine act as an acid or base?
At pH 6.00 glycine acts as a buffer, because at pH 6.00 the condition is acidic and glycine is an amino acid, so its zwitterion forms an equilibrium, which means there is a positive charge on the N of the amino group and a negative charge on the carboxylate.
Is amino acid a strong or weak acid?
They are quite soluble in water but insoluble in non-polar solvents like benzene or ether. Their acidic and basic properties are exceptionally weak for molecules that contain an acid carboxyl group and a basic amino group.
Is glycine hydrochloride a weak acid?
Glycine hydrochloride is the amino acid glycine adsorving a molecule of Hydrochloric acid, wich is a strong acid.
Is glycine an acid?
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids.
Why glycine is strong acidic than acetic acid?
The acid of glycine is more acidic than acetic acid, due to the electron-‐withdrawing effect of the ammonium ion. The amine of glycine is more basic than that of glycine methyl ester. However, glycine's amino group is less basic than aliphatic amines, due to the electron-‐ withdrawing effect of the carboxylic acid.
Is glycine neutral?
(iii) Comparison between energies of the microsolvated neutral and zwitterion glycine/silica cases reveals that for n = 0 and 1 glycine sticks as a neutral form, for n = 2 neutral and zwitterions are almost degenerate, and for n = 3−5 the zwitterionic case is largerly the preferred one.
Are amino acids weak acids?
One of these compounds is a weak acid; the other is a weak base. Thus, it is not surprising that an H+ ion is transferred from one end of the molecule to the other when an amino acid dissolves in water....Amino Acids.The Amino AcidsZwitterionsThe Amino Acids Used to Synthesize ProteinsThe Acid-Base Chemistry of the Amino Acids
What is true glycine?
Glycine is a large, polar amino acid. Glycine increases the flexibility of the peptide backbone. Glycine is similar to tyrosine in terms of structure and chemical properties. Glycine is special because it contains a -SH group important in protein folding.
What is the pH of glycine?
pH 2.2Glycine (0.1 M, pH 2.2)
Why is glycine the simplest amino acid?
Glycine is the simplest amino acid with an amino and a carboxyl group. Its chemical formula is NH2‐CH2‐COOH. It lacks any R or variable groups and hence considered the simplest amino acid among the 20 types of amino acids available.
What is the conjugate acid of glycine?
GlyciniumGlycinium is an alpha-amino-acid cation that is the conjugate acid of glycine, arising from protonation of the amino. It has a role as a fundamental metabolite. It is a conjugate acid of a glycine.
What is the function of glycine in the brain?
Glycine also helps regulate nerve impulses in the central nervous system, most specifically those of the spinal cord, retina, and the control center of the brain known as the brainstem . Glycine will also bind with toxic substances and aid in their excretion from the body.
How long after stroke can you take glycine?
Early research published in the journal Cerebrovascular Disease suggested that a sublingual (under the tongue) dose of glycine given within six hours of a stroke could limit the damage done to the brain. 3 .
What is the association between low glycine levels in the blood and the onset of insulin resistance?
Insulin Resistance. There is a known association between low glycine levels in the blood and the onset of insulin resistance. People with insulin resistance are unable to use insulin effectively , leading to high blood sugar levels and the onset of type 2 diabetes .
What is the amino acid that makes up collagen?
Glycine is an amino acid that functions as a building block for certain proteins, most especially the collagen found in skin, ligaments, muscles, bones, and cartilage. It makes up around 35 percent of the collagen in the human body.
Does glycine help with ulcers?
When applied as a topical cream, glycine may help promote the healing of certain types of leg ulcers. Much of the research dates back to the 1980s when it was found that a topical cream containing glycine helped treat leg ulcers caused by rare disorders such as prolidase deficiency and Klinefelter syndrome .
Does glycine help with cognitive impairment?
A 2016 review of studies reported that glycine supplements taken with antipsychotic therapy reduced the incidence of cognitive and physiological side effects by 34%. 2 To do so, however, required relatively high doses (8 milligrams or more) in order for glycine to pass through the blood-brain barrier . This is problematic since high doses can ...
Can you take glycine for a stroke?
Glycine is sometimes prescribed to people who have just had an ischemic stroke. Ischemic strokes occur when the arteries to the brain become narrowed or blocked, causing the restriction of blood flow (ischemia) to the brain. The evidence in support of its use has been mixed and often contradictory.
What are the weak acids?
The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid (HF).
What is the difference between a strong and weak acid and a concentrated acid?
Be careful not to confuse the terms strong and weak with concentrated and dilute. A concentrated acid is one that contains a low amount of water. In other words, the acid is concentrated. A dilute acid is an acidic solution that contains a lot of solvent.
How to tell if an acid is strong or weak?
Distinguishing Between Strong and Weak Acids. You can use the acid equilibrium constant K a or pK a to determine whether an acid is strong or weak. Strong acids have high K a or small pK a values, weak acids have very small K a values or large pK a values. Strong and Weak Vs. Concentrated and Dilute.
Can you drink acetic acid?
You can drink diluted acetic acid (the acid found in vinegar), yet drinking the same concentration of sulfuric acid would give you a chemical burn. The reason is that sulfuric acid is highly corrosive, while acetic acid is not as active. While acids tend to be corrosive, the strongest superacids (carboranes) are actually not corrosive and could be held in your hand. Hydrofluoric acid, while a weak acid, would pass through your hand and attack your bones .
Is 0.0005 a dilute acid?
No matter how much water you remove, that will be true. On the flip side, a 0.0005 M HCl solution is dilute, yet still strong. Strong Vs.
Is HF a strong acid?
Weak Acids. Weak acids do not completely dissociate into their ions in water. For example, HF dissociates into the H + and F - ions in water, but some HF remains in solution, so it is not a strong acid. There are many more weak acids than strong acids. Most organic acids are weak acids.
What is the difference between strong and weak acids?
The main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids partially dissociate in aqueous solutions.
What is weak acid?
Weak acids are molecules that partially dissociate into ions in aqueous solutions. Weak acids do not release all the H + ions to the solution. The acid dissociation constant (K a) is a small value than that of strong acids. The pH of the solution is about 3-5.
How is the strength of an acid determined?
The strength of an acid is determined by the polarity and the atomic sizes of the acid molecule. According to the way that acid molecules dissociate in water, there are two types of acids as strong acids and weak acids. The main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak ...
What is a strong acid?
Definition. Strong Acid: Strong acids are molecules that completely dissociate into their ions when it is in water. Weak Acid: Weak acids are molecules that partially dissociate into ions in aqueous solution.
How do acids release H+ ions?
In other words, acids release H + ions into the solution by their complete ionization. The strength of an acid is characterized by their acid dissociation constant values (K a ). Normally, strong acids have a very large K a value. The stronger the acid, more easily it loses protons.
Why is pH influenced by acid?
On the other hand, the pH of the solution is greatly influenced by strong acids because strong acids release H+ ions to the solution. The pH depends on the H+ concentration. The relationship between H+ concentration and the pH can be given as below. If the acid is a strong acid, the pH value is a very small value.
Does a weak acid raise the H+ concentration?
That is because the weak acid does not raise the H+ concentration of a solution as a strong acid does. In a system of weak acid in water, there are H+ ions, anion of the molecule and the weak acid molecule present in the solution. For example, the dissociation of ethanoic acid can be shown as below. Figure 1: Dissociation of Ethanoic Acid.

Overview
Physiological function
The principal function of glycine is it act as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction with hydroxyproline. In the genetic code, glycine is coded by all codons starting with GG, namely GGU, GGC, GGA and GGG.
History and etymology
Glycine was discovered in 1820 by the French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid. He originally called it "sugar of gelatin", but the French chemist Jean-Baptiste Boussingault showed that it contained nitrogen. The American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig, proposed the name "glycocoll"; however, the Swedish chemist Berzelius suggested the simpler name "glycine". The name comes f…
Production
Although glycine can be isolated from hydrolyzed protein, this is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis. The two main processes are amination of chloroacetic acid with ammonia, giving glycine and ammonium chloride, and the Strecker amino acid synthesis, which is the main synthetic method in the United States and Japan. About 15 thousand tonnes are produced annually in this way.
Chemical reactions
Its acid–base properties are most important. In aqueous solution, glycine is amphoteric: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about 9.6, it converts to glycinate.
Glycine functions as a bidentate ligand for many metal ions, forming amino acid complexes. A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which …
Metabolism
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which ma…
Presence in space
The presence of glycine outside the earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft Stardust from comet Wild 2 and subsequently returned to earth. Glycine had previously been identified in the Murchison meteorite in 1970. The discovery of glycine in outer space bolstered the hypothesis of so called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the univer…