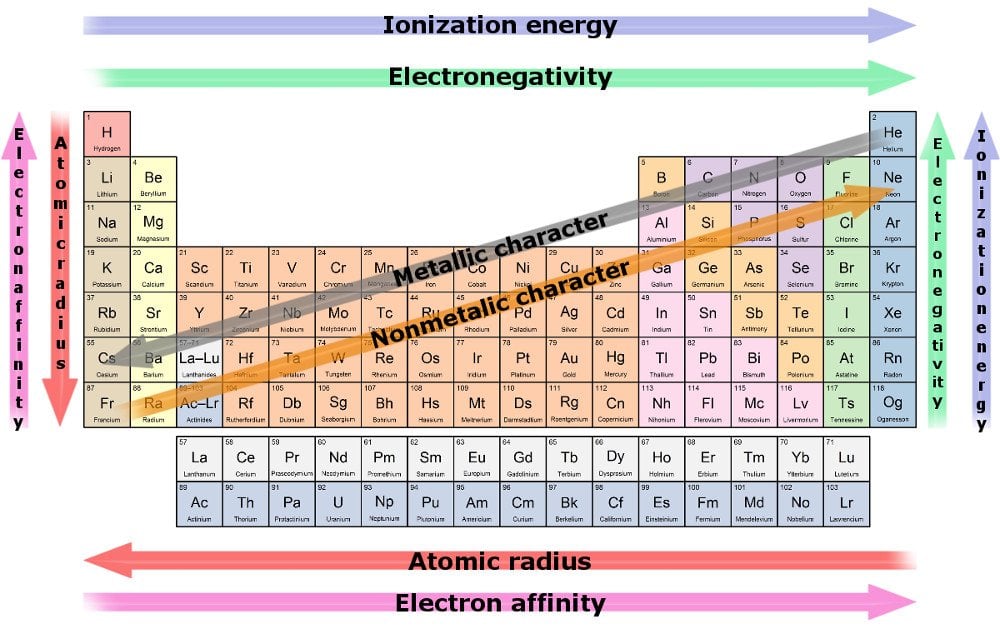
The reactivity of group 13 elements towards oxygen increases down the group. Boron is unreactive towards oxygen in its crystalline form. Finely divided amorphous boron
Boron
Boron is a chemical element with the symbol B and atomic number 5. Produced entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, it is a low-abundance element in the Solar system and in the Earth's crust. Boron is concentrated on Earth by the water-solubility of it…
What are the 14 groups of elements in the periodic table?
1 Group 1: Alkali metals group (hydrogen not included) 2 Group 2: Alkaline earth metals group 3 Group 3-12: Transition and Inner transition metals group 4 Group 13: Boron group 5 Group 14: Carbon group 6 Group 15: Nitrogen group 7 Group 16: Oxygen group 8 Group 17: Halogen group 9 Group 18: Noble gases group
What is the most reactive group on the periodic table?
the most reactive (non-metals) group in the periodic table is GROUP 17+they are all reactive What metals are the most reactive group of the periodic table? Metals are placed on left side on the periodic table.
What is the reactivity of boron in Group 13?
Moreover, all of the other elements in group 13 are relatively reactive at moderate temperatures, while boron's reactivity only becomes comparable at very high temperatures. One characteristic that all do have in common is having three electrons in their valence shells.
What group do transition metals belong to on the periodic table?
So they are included in group 3. But as these elements have few different properties, they are grouped as separate elements known as inner transition elements. For detailed information on transition and inner transition metals, read the main articles on Transition metals on periodic table and Inner transition metals on periodic table.
Why is group 13 less reactive?
Reactions and Compounds of the Heavier Group 13 Elements The halides of the heavier metals (In and Tl) are less reactive with water because of their lower charge-to-radius ratio. Instead of forming hydroxides, they dissolve to form the hydrated metal complex ions: [M(H 2O) 6] 3+.
What is group 13 on the periodic table?
boron group elementboron group element, any of the six chemical elements constituting Group 13 (IIIa) of the periodic table. The elements are boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh).
What is the nature of group 13 elements?
The group 13 elements never react with hydrogen because the valency of hydrogen is one and that of the boron family is three. The trihalides of group 13 elements are strong Lewis acids because they have the tendency to form compounds with electron-pair donors, the Lewis bases.
Is the boron family Reactive or nonreactive?
unreactiveReactions and Compounds of Boron. Elemental boron is a semimetal that is remarkably unreactive; in contrast, the other group 13 elements all exhibit metallic properties and reactivity.
What is a group of 13 called?
Group 13 is also known as the Boron group.
How reactive is the boron family?
Chemical reactivity Boron, the first element in the group, is generally unreactive with many elements except at high temperatures, although it is capable of forming many compounds with hydrogen, sometimes called boranes.
What are the trends In group 13?
Each of the Group 13 metals forms both covalent compounds and ionic coordination complexes. All of the Group 13 (IIIA) elements have a valence shell electron configuration of ns2np1. As a consequence all of the Group 13 elements for compounds in which they adopt a +3 oxidation state.
Is group 13 metal or nonmetal?
Group 13: Chemical Reactivity. The boron family contains the semi-metal boron (B) and metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). The boron family contains the semi-metal boron (B) and metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl).
Are group 13 elements metals?
This group includes boron, aluminum, gallium, indium, thallium, and ununtrium (B, Al, Ga, In, Tl, and Uut, respectively). These elements all have three valence electrons. Boron is the only metalloid in this family. The rest of the elements are considered to be poor metals.
Is boron reactive or stable?
Boron is a poor conductor of electricity, and is fairly non-reactive, although it is water soluble. The most common uses for boron-containing compounds includes a bleach for clothing, a swimming pool disinfectant and to produce green flames.
Are boron metals reactive?
Aluminium and gallium oxides are amphoteric in nature. Whereas indium and thallium are basic in nature. Reactivity towards acids and alkalis: Boron is unreactive when comes in contact with acids and alkalis at moderate temperatures.
What is the difference between group 3 and group 13?
Answer: Boron and Aluminium are present in Group 13 of the modern periodic table. Group 13 (IUPAC System) can also be referred to as Group IIIA. Logically, Boron and Aluminum can't be placed alongwith elements such as Yttrium as they don't exhibit properties of a transition metal.
Is group 13 metal or nonmetal?
Although group 13 includes aluminum, the most abundant metal on Earth, none of these elements was known until the early 19th century because they are never found in nature in their free state.
Why is group 13 called the boron group?
Group 13 of the periodic table is also called the boron group because boron (B) is the first element at the top of the group (see Figure below). Boron is also the only metalloid in this group. The other four elements in the group—aluminum (Al), gallium (Ga), indium (In), and thallium (Tl)—are all metals.
What charge does group 13 have?
If you look at the periodic table, you will find the metals in groups (from one to 16). Group one is composed of metals that have a +1 charge, while all the metals in groups 2,3,4,5,6,7,8,9,10,11,12, and 16 have a charge +2. Then, metals in groups thirteen and fifteen have a charge of +3.
Is boron a metal or nonmetal or metalloid?
Boron is classified as a metalloid, having properties of both metals and nonmetals: it and conducts electricity at high temperatures; but at room temperature, is it an insulator.
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
What is the oxygen group on the periodic table?
Oxygen group is the group 16 on the periodic table.
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Which group is alkaline earth metals?
Alkaline earth metals are the group 2 elements on the periodic table.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is the reactivity of an element?
Reactivity of Elements of the Periodic Table. Reactivity is a measure of how easily an element will combine with other elements to form compounds. Some elements are unreactive and need energy putting in others will react spontaneously and easily.
Why do some elements react spontaneously?
Some elements are unreactive and need energy putting in others will react spontaneously and easily. The size of the nucleus determines the chemical reactivity of the element due to its ability to hold onto electrons and attract electrons. Patterns of reactivity vary depending on the size of the nucleus, the number of electrons and the number ...
Which element is unreactive?
Most of the elements in the boron group show increasing reactivity as the elements get heavier in atomic mass and higher in atomic number. Boron, the first element in the group, is generally unreactive with many elements except at high temperatures, although it is capable of forming many compounds with hydrogen, sometimes called boranes. The simplest borane is diborane, or B 2 H 6. Another example is B 10 H 14 .
Which element is notorious for violating the octet rule?
Although situated in p-block, the group is notorious for violation of the octet rule by its members boron and (to a lesser extent) aluminium. These element may place only six electrons (in three molecular orbitals) onto valence shell. All members of the group are characterized as trivalent .
What is the boron group?
The boron group are the chemical elements in group 13 of the periodic table, comprising boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and perhaps also the chemically uncharacterized nihonium (Nh). The elements in the boron group are characterized by having three valence electrons. These elements have also been referred ...
What are the trends in the borion group?
There are several trends that one could notice as they look at the properties of Boron group members. The Boiling Points of these elements drop from period to period, while densities tend to rise.
Who discovered thallium?
Thallium, the heaviest stable element in the boron group, was discovered by William Crookes and Claude-Auguste Lamy in 1861. Unlike gallium and indium, thallium had not been predicted by Dmitri Mendeleev, having been discovered before Mendeleev invented the periodic table.
Is fluorine a stable element?
Fluorine, the first halogen, is able to form stable compounds with every element that has been tested (except neon and helium ), and the boron group is no exception. It is even hypothesized that nihonium could form a compound with fluorine, NhF 3, before spontaneously decaying due to nihonium's radioactivity.
Which group of elements has the most inert s-pair effect?
The inert s-pair effect is significant in the group-13 elements, especially the heavier ones like thallium. This results in a variety of oxidation states. In the lighter elements, the +3 state is the most stable, but the +1 state becomes more prevalent with increasing atomic number, and is the most stable for thallium.