Full Answer
What is the chemical name of H2Cr2O7?
What is the name of H2Cr2O7? What is the chemical name of H2Cr2O7? Dichromic acid is the chemical name of H2Cr207. What is the formula for dichromic acid? What is the formula dichromic acid? What is the acid used in chromic acid? Chromic acid is the acid in chromic acid.
Is H2Cr2O7 a Lewis acid or base?
It is a Lewis acid and can react with a Lewis base, such as pyridine in a non-aqueous medium such as dichloromethane ( Collins reagent ). Dichromic acid, H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid.
What is the name of the compound with formula H2CrO4?
Chromic Acid is a naturally occurring oxide with a formula H 2 CrO 4. Chromic Acid is also called Tetraoxochromic acid or Chromic (VI) acid. It is usually a mixture made by adding concentrated sulphuric acid (H 2 SO 4) to a dichromate which consists of a variety of compounds and solid chromium trioxide.
What are the properties of chromic acid?
Properties of Chromic acid – H2CrO4 Chromic acid H 2 CrO 4 Molecular Weight of Chromic acid 118.008 g/mol Density of Chromic acid 1.201 g/cm 3 Melting Point of Chromic acid 197 °C Boiling Point of Chromic acid 250 °C
How to protect yourself from a toxic substance?
Is dichromic acid a conjugate acid?
About this website
What type of acid is H2Cr2O7?
Substance Details - Chromic acid (H2Cr2O7)
Is H2CrO4 a strong acid?
Molecular chromic acid – H2CrO4 is similar to sulphuric acid (H2SO4) as both are strong acids, however, only the first proton is lost easily. Dichromic acid – H2Cr2O7 is the fully protonated form of the dichromate (Cr2O7–) ion.
Is chromic acid a strong oxidizing agent?
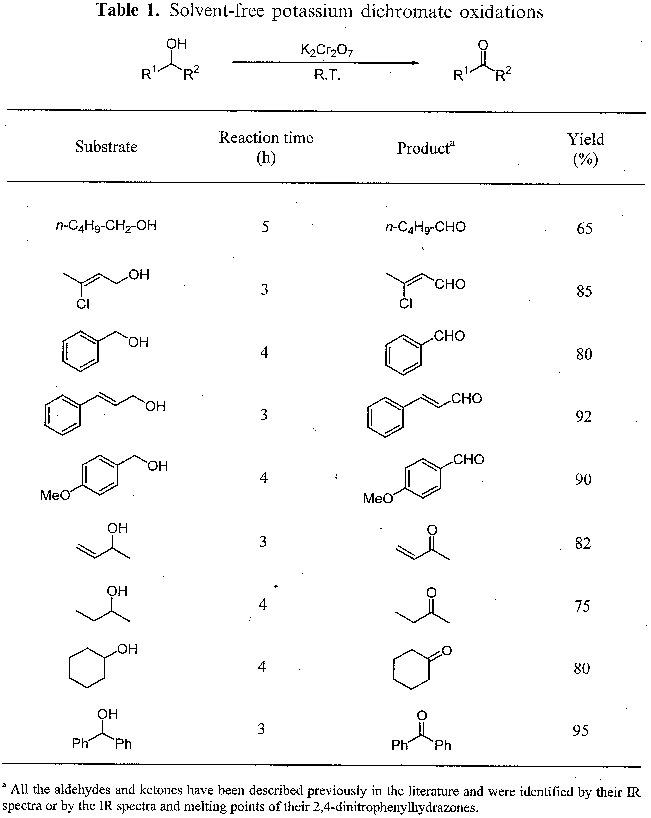
Chromic acid is a strong oxidizing agent, used to oxidize many classes of organic compounds, the most common of which is alcohols.
What is the name of this compound H2Cr2O7?
Dichromic acidDichromic acidPubChem CID26090Molecular FormulaCr2H2O7SynonymsDichromic acid 13530-68-2 Dichromic(VI) acid Chromic acid (H2Cr2O7) Dichromic acid (H2Cr2O7) More...Molecular Weight218.00DatesModify 2022-09-17 Create 2004-09-163 more rows
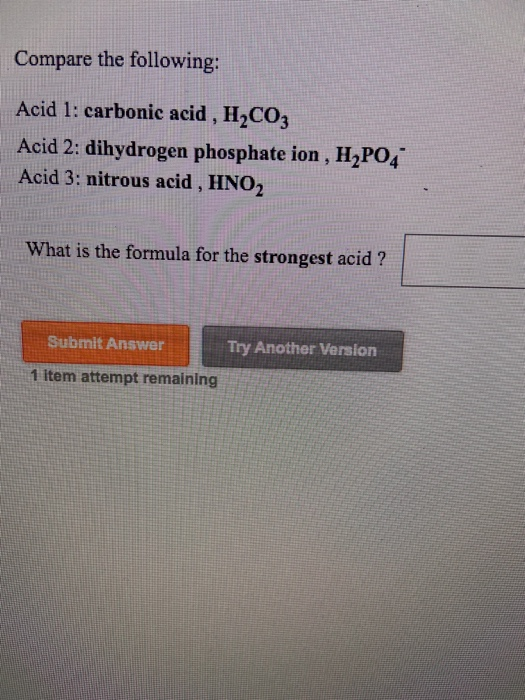
Which of the following is the strongest acid?
So strongest acid is CCl3COO−.
What is H2CrO4 called?
Chromic acid | H2CrO4 - PubChem.
Which is the strongest oxidizing agent?
FluorineFluorine is the best oxidising agent, with the highest positive electrode potential value.
What is a weak oxidizing agent?
Weak oxidizing agents will react less vigorously than a strong oxidizing agent, but can still participate in reactions that generate heat and possibly gaseous products which can pressurize a closed container, and which may go on to participate in further reactions.
Which is the strongest reducing agent?
Lithium is the strongest reducing agent because of lower reduction potential (i.e it has lower tendency to acquire electrons.)
Is hmno4 a strong acid?
As a strong acid, HMnO4 is deprotonated to form the intensely purple coloured permanganates. Potassium permanganate, KMnO4, is a widely used, versatile and powerful oxidising agent.
Is chromic acid a strong acid?
Chromic acid (H2CrO4) Chromic acid is a very weak acid and its salts can be dissociated even by acetic acid. It has a strong oxidising action and is itself reduced to CrO3; because of this, it should never be used in combination with alcohol or formalin.
Is chromate an acid?
Chromates are the salts of chromic acid which contains the chromate anion with the chemical formula CrO42– and usually have an intense yellow color. Chromate is the oxoanion which results from the removal of protons from chromic acid. It is also called chromium oxoanion or divalent inorganic anions.
Is C2H5NH2 a strong or weak acid or base?
Ethylamine, C2H5NH2, is a weak base.
What does H2CrO4 reagent do?
H2CrO4 As A Reagent For The Oxidation Of Alcohols Once H2CrO4 is formed, its reactions are pretty straightforward: it converts primary alcohols (and aldehydes) to carboxylic acids and secondary alcohols to ketones.
What does H2CrO4 do in a reaction?
Chromic acid (H2CrO4) oxidizes alcohols in aqueous solutions of sodium dichromate. It reacts with alcohols to form a chromic ester in which the alcohol oxygen atom bridges the carbon and chromium atoms. Thus, the ester forms by nucleophilic attack of the alcohol's oxygen atom on the chromium atom.
Is H2SO4 a strong acid?
Sulfuric Acid (H2SO4, oil of vitriol) is a strong acid that is soluble in water. It is one of the most abundantly produced chemicals for industrial use including fertilizer production, ore processing, and oil refining. Sulfuric acid,HSO4, is a molecule that contains sulfur,hydrogen and oxygen.
Dichromic acid: characteristics, formula, uses and warnings
Dichromic acid is an unstable, oxidising, dibasic acid known only as a solution and in the form of orange or red dichromatic salts (such as potassium dichromate). Its chemical formula is H2Cr2O7, and it can be produced when two chromic acid molecules lose a water molecule, as seen below. It is hygroscopic, meaning it absorbs […]
Chemical Equation Balancer
Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured.
Homework # 7, Graded Answers - Ventura College Chemistry, Malia Rose-Seisa
Homework # 7, Graded Answers Chem20, Elementary Chemistry 6.55) A sugar crystal contains approximately 1.8 1017 sucrose (C 12 H 22 O 11) molecules. What is its mass in milligrams?
What is a strong acid?
A strong acid is one which completely dissociates in its solvent. Under most definitions, the acid dissociates into a positively-charged hydrogen ion (proton) and a negatively-charged anion.
What is the name of the acid that is colorless and has a pungent odor?
Hydrochloric acid: Hydrochloric acid also goes by the name of muriatic acid. The acid is colorless and has a pungent odor. Humans and most other animals secrete hydrochloric acid in the digestive system. The acid has many commercial applications. It is used to produce inorganic compounds, refine metals, pickle steel, and regulate pH. Of the common strong acids, it is one of the least hazardous to handle, least expensive, and easiest to store.
What is the purpose of nitric acid?
While colorless in pure form, nitric acid yellows over time as it decomposes into nitrogen oxides and water. In chemistry, one of its key uses is for nitration. This is where a nitro group gets added to a molecule (usually organic). Nitric acids finds use as an oxidant in nylon production, as the oxidizer in rocket fuel, and as an analytical reagent.
How much dissociation is a strong acid?
As the strong acids become more concentrated, they may be unable to fully dissociate. The rule of thumb is that a strong acid is 100 percent dissociated in solutions of 1.0 M or less. Cite this Article. Format.
What makes an acid strong?
What makes them "strong" is the fact that they completely dissociate into their ions (H + and an anion) when they are mixed with water. Every other acid is a weak acid. Because there are only seven strong acids, it is easy to commit the list to memory. Note that some chemistry instructors may refer only to six strong acids.
Is S a solvent?
Here, S is a solvent molecule, such as water or dimethyl sulfoxide (DMSO).
Are Strong Acids Always Strong?
As the strong acids become more concentrated, they may be unable to fully dissociate. The rule of thumb is that a strong acid is 100 percent dissociated in solutions of 1.0 M or lower concentration .
What is chromic acid?
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, ...
What is the compound that is produced from chromic acid?
Chromyl chloride, CrO 2 Cl 2 is a well-defined molecular compound that is generated from chromic acid.
What is the protonated form of dichromate?
Dichromic acid . Dichromic acid , H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid. Dichromic acid will behave the same exact way when reacting with an aldehyde or ketone.
How is chromic acid made?
Molecular chromic acid could in principle be made by adding chromium trioxide to water ( cf. manufacture of sulfuric acid ).
Why is sulfuric acid used in glass cleaners?
Because a solution of chromic acid in sul furic acid (also known as a sulfochromic mixture or chromosulfuric acid) is a powerful oxidizing agent, it can be used to clean laboratory glassware, particularly of otherwise insoluble organic residues. This application has declined due to environmental concerns.
Why is chromic acid used in brass?
Chromic acid was widely used in the musical instrument repair industry, due to its ability to "brighten" raw brass. A chromic acid dip leaves behind a bright yellow patina on the brass. Due to growing health and environmental concerns, many have discontinued use of this chemical in their repair shops.
What is the p-ka of equilibrium?
The p Ka for the equilibrium is not well characterized. Reported values vary between about −0.8 to 1.6. The value at zero ionic strength is difficult to determine because half dissociation only occurs in very acidic solution, at about pH 0, that is, with an acid concentration of about 1 mol dm −3. A further complication is that the ion [HCrO 4] − has a marked tendency to dimerize, with the loss of a water molecule, to form the dichromate ion, [Cr 2 O 7] 2− :
What is the formula for chromatic acid?
What is Chromic acid? Chromic Acid is a naturally occurring oxide with a formula H 2 CrO 4. Chromic Acid is also called Tetraoxochromic acid or Chromic (VI) acid. It is usually a mixture made by adding concentrated sulphuric acid (H 2 SO 4) to a dichromate which consists of a variety of compounds and solid chromium trioxide.
How is chromic acid formed?
While applying sulphuric acid to the paste and continuously mixing, chromic acid is formed.
What are the uses of chromic acid?
This compound is widely used as an intermediate in chromium plating. Chromic acid is also used in coloured glass and ceramic glazes. In the 1940s, this compound was an integral part of several hair dyes.
What is chromosulfuric acid used for?
It is used in ceramic glazes, coloured glass. Chromosulfuric acid or Sulfochromic mixture is a strong oxidizing agent that is used to clean laboratory glassware. It has the ability to brighten raw brass and therefore it is used in the instrument repair industry. In the year 1940, it was used in hair dye.
What is the best way to treat a chromatic acid?
In case of any burns caused by this acid, it is treated with a dilute solution of sodium thiosulfate.
Can dichromic acid react with aldehyde?
When reacting with an aldehyde or ketone, Dichromic acid can behave the same exact way. However, the caveat to this argument is that no more than a ketone will oxidise a secondary ketone and dichromic acid will only oxidise the aldehyde.
Is aldehyde a ketone?
For the first step of the mechanism, the aldehyde would be oxidised to a ketone and oxidised to a carboxylic acid again, subject to no major steric hindrance impeding this reaction. Chromic acid is capable of oxidising many forms of organic compounds, and many variants have been created for this reagent.
1.2 3D Status
Conformer generation is disallowed since MMFF94s unsupported element, MMFF94s unsupported atom valence, mixture or salt
2.1 Computed Descriptors
disodium;zinc;oxido- (oxido (dioxo)chromio)oxy-dioxochromium;dichloride
How to protect yourself from a toxic substance?
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]: Ensure that medical personnel are aware of the material (s) involved and take precautions to protect themselves . Move victim to fresh air. Call 911 or emergency medical service. Give artificial respiration if victim is not breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device. Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. In case of contact with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact, avoid spreading material on unaffected skin. Keep victim calm and warm. Effects of exposure (inhalation, ingestion or skin contact) to substance may be delayed. (ERG, 2016)
Is dichromic acid a conjugate acid?
2004-09-16. Dichromic acid is a chromium oxoacid. It is a conjugate acid of a hydrogen dichromate. Chromic acid, solution appears as a dark red aqueous liquid. Corrosive to metals and tissue.