What makes a molecule polar or non polar?
- An electronegativity difference between two atoms that is less than 0.5 results in a nonpolar-covalent bond.
- Electronegativity differences between two atoms from 0.5 to 2.0 result in a polar-covalent bonds.
- An electronegativity difference between two atoms that is greater than 2.0 generally results in an ionic bond.
Is H2SO4 polar or non polar?
The H2SO4 molecule is polar in nature because of the bent H-O-S bonds which are present in it. As a result, the charge distribution across the molecule becomes non-uniform. It’s the process in which atomic orbitals fuse to form new hybridized orbitals, which in turn influences molecular geometry. The new orbitals are known as hybridized orbitals.
Is H2O an atom or molecule?
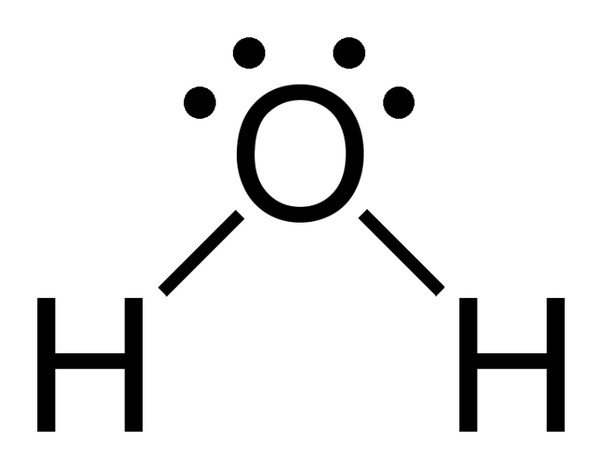
The chemical formula for water is H2O, which means each molecule of water consists of one oxygen atom chemically bonded to two hydrogen atoms. Thus, water is a compound. It’s also a molecule, which is any chemical species formed by two or more atoms chemically bonded to each other. Is a water molecule water?
How does molecule that has polar bonds be nonpolar?
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
.png)
What determines the polarity of a molecule?
The polarity of any molecule depends on these following factors: Its shape. the difference of electronegativities between the atom and. the net dipole moment in the molecule. Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen ...
Is H2O a polar molecule?
Yes, H2O or water is a polar molecule and a polar solvent as well. The molecule is made up of two hydrogen atoms and one oxygen atom. To understand why this molecule is polar and nonlinear keep reading this article.
What direction does the dipole moment go?
As a result of this pull, the dipole moment’s direction will be from Hydrogen towards the Oxygen atom on both sides . As a result of the dipole moment on both sides, there is a net dipole moment in this molecule. This would have been avoided if the shape of the molecule was linear.
What is the difference between oxygen and hydrogen?
Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen and Hydrogen’s electronegativities, it is much higher than 0.5, making the O-H bond a polar covalent bond.
Why is water a unique molecule?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
Why do both hydrogen atoms take up a position like the one shown in the figure?
Both the Hydrogen atoms take up a position like the one shown in the figure to keep the repulsive forces at the minimum. Due to this arrangement of both Hydrogen atoms the shape of the molecule is bent making the geometry of this molecule nonlinear.
Why are the charges near the oxygen atom slightly negative?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
How to determine if a molecule is polar or nonpolar?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might not have any overall polarity, depending upon its shape. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers lie at the same point in space, then the molecule has no overall polarity (and is non polar). If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. A molecule can only be polar if the structure of that molecule is not symmetric.
How to determine polarity of a molecule?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule. A molecule that contains polar bonds, might not have any overall polarity, depending upon its shape. The simple definition of whether a complex molecule is polar or not depends upon whether its overall centers of positive and negative charges overlap. If these centers lie at the same point in space, then the molecule has no overall polarity (and is non polar). If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule
How many dipoles does CO2 have?
Think of this, it is linear, it 4 dipoles from the Oxygen atoms but, the carbon atom is able to balance out the polarity from those four dipoles. If you tried to run electricity through a chain of CO2 molecules, you would fail. Also, CO2 is not magnetic.
What is a nonpolar covalent bond?
Bonds that are partly ionic are called polar covalent bonds. Nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. The result is a bond where the electron pair is displaced toward the more electronegative atom.
Why is the shape of a molecule not linear?
The reason the shape of the molecule isn't linear and nonpolar (e.g., like CO2) is because of the difference in electronegativity between hydrogen and oxygen. The electronegativity value of hydrogen is 2.1, while the electronegativi
Why is H2O polar?
Water (H2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding. When solutes are added to water, they may be affected by the charge distribution.
What does it mean when all elements have different electronegativities?
So first of all elements in the periodic table have different electronegativities which sounds like something complicated but just means how much they can PULL electrons towards them. If you look across the periodic table you can see a trend. As you go down the group the electronegativity decreases and as you go go to right (so through the periodes) the electronegativity increases. That's due to the so called octet rule which seems to be a very stable