Full Answer
Is iron (II) oxide ionic or covalent?
An ionic compound is neutral, so the positive and negative charges are equal. In the case of iron(II) oxide, one #"Fe"^(2+)"# ion combined with one #"O"^(2-)"# ion creates a neutral compound. #2^+ + 2^(-) = 0#.
What is the formula for iron (II) oxide?
Iron(II) oxide has the Roman numeral II indicating it is the Fe+2 ion, which has lost two electrons. When an oxygen atom takes two electrons it becomes an oxide ion with the formula O-2. The two ions combine to form iron(II) oxide with the formula FeO.
Is Fe2O3 ionic or covalent?
Is Fe2O3 Ionic or Covalent? (Detailed Explanation) Iron (III) oxide or ferric oxide is an inorganic compound with the molecular formula Fe 2 O 3. Fe 2 O 3 has a molecular mass of 159.68 g/mol and is often known as rust, having a reddish-brown color. In this article, we will understand the bonding between the atoms of Fe 2 O 3 molecules.
What is the mineral form of iron oxide?
Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron (III) oxide (ferric oxide).
What is iron oxide?
Where is iron oxide found?
What is the formula for FeO?
How many electrons does an oxygen atom need to fill its outer shell?
How many electrons does iron need to oxidize?
What is the Roman numeral for iron oxide?
What is the color of water when it attaches to iron?
See 4 more
About this website

Is iron II oxide ionic or covalent?
ionic compoundIron (II) oxide is an ionic compound that is also known as ferrous oxide or iron monoxide. FeO is the chemical or molecular formula of Iron (II) Oxide, i.e., it consists of one iron atom and one oxygen atom.
Is iron oxide a ionic bond?
Iron Oxide (Fe2O3) also known as rust is also an ionic bond, it has metallic compound Iron (Fe) which contains positively charged cations and it has nonmetallic compound Oxygen (O) which contains negatively charged anions.
Is Fe2O3 an ionic or covalent compound?
ionic bondHence, Fe 2 O 3 is an ionic bond, not a covalent.
Why is Fe2O3 an ionic compound?
According to the definition of ionic compounds, Fe2O3is an ionic compound as the ionic bond is formed between a metal and a nonmetal. And as there is a bond formed between Fe, a metal, and O, a nonmetal, in Fe2O3, hence an ionic compound.
What type of compound is iron oxide?
inorganic compoundIron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3.
What kind of bond is iron oxide?
ionicThe bond formed between iron and oxygen is due to the difference in electronegativity between the two atoms. Since iron is metal and oxygen is non-metal therefore the bonding between oxygen and iron is ionic.
Is it ionic or covalent?
There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences.
Is Fe ionic or covalent or metallic?
Answer and Explanation: Option d (metallic) is the correct answer. Iron (Fe) has an atomic number of 26 and is found on the left side of the periodic table in the transition metal groups. As it is a transition metal, it exhibits metallic bonding.
Is fe2o3 a element or compound?
Fe2O3 is an inorganic compound with the chemical name Iron(III) oxide. It is also known as Hematite or Red iron oxide.
Is iron oxide a covalent compound?
Iron is a metal and oxygen (oxide) is a nometal; therefore, iron(III) oxid is an ionic compound.
Is FeO a covalent compound?
Yes, FeO is polar covalent compound. In FeO structure, the iron atom has an electronegativity of 1.83 and the oxygen atom has electronegativity of 3.44.
What are the ions in Fe2O3?
The Lewis Structure of iron (III) oxide, Fe2O3, consists of five ions: Two iron ions with a +3 charge each, and three oxygen ions with a -2 charge each. Iron III oxide is an ionic compound, because it consists of a metal and non-metal.
Is iron oxide a covalent compound?
Iron is a metal and oxygen (oxide) is a nometal; therefore, iron(III) oxid is an ionic compound.
What type of bond is FeO?
In FeO structure, the iron atom has an electronegativity of 1.83 and the oxygen atom has electronegativity of 3.44. The difference between both electronegativities are 3.44 (O) – 1.83 (Fe) = 1.61. The nature of bond in FeO is polar covalent thus the difference is very near to that of ionic bonds.
Is FeO an ionic compound?
Iron(II) oxideIron(II) oxide / IUPAC ID
Is Fe ionic or covalent or metallic?
Answer and Explanation: Option d (metallic) is the correct answer. Iron (Fe) has an atomic number of 26 and is found on the left side of the periodic table in the transition metal groups. As it is a transition metal, it exhibits metallic bonding.
What is Fe3O4 called?
Fe_3O_4 is iron (II,III) oxide. Its common name is magnetite. It is not the same as iron (II) oxide as it is made up of iron (II) and iron (III)...
Why is FeO named iron II oxide?
FeO is the molecular formula for iron (II) oxide. Because iron forms more than one cation, or positive ion, the Roman numeral II is used to distin...
Is FeO an acid?
An acid is characterized by the ability to give up hydrogen ions. Since there are no hydrogen ions to give in FeO, it is not considered an acid....
Iron II Oxide | Formula, Uses & Color | Study.com
Learn about what iron II oxide is and how it is formed. Understand the iron II oxide formula, properties and uses, and see an iron II oxide...
What is the formula for iron(II) oxide? | Socratic
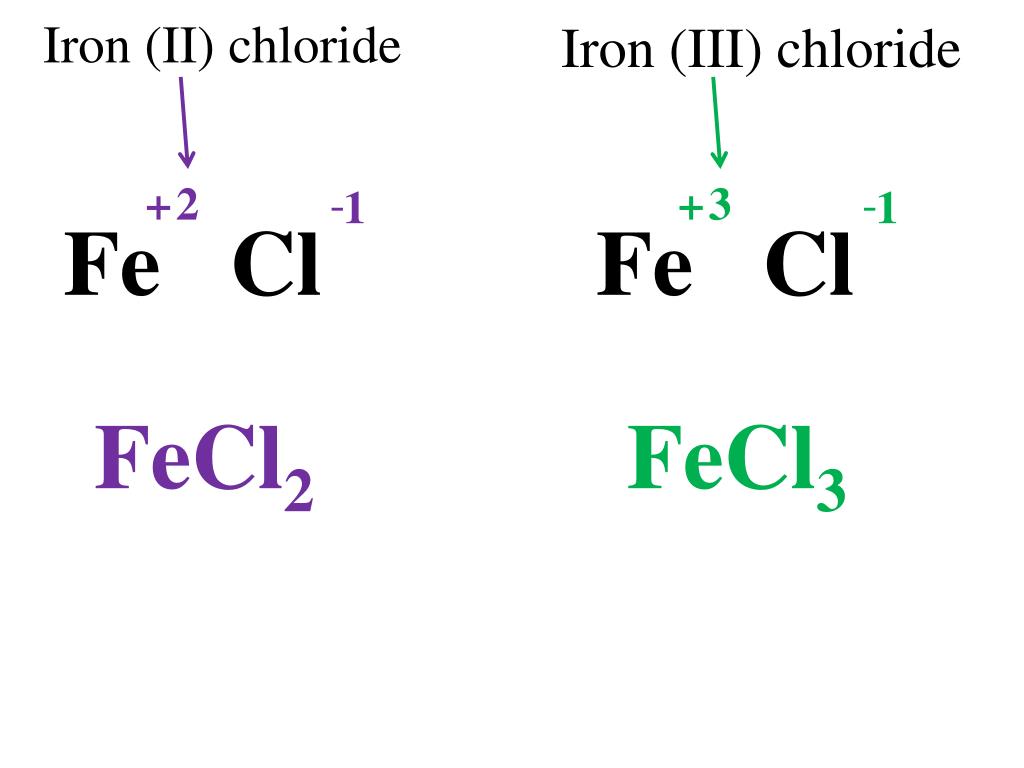
FeO Iron II has a positive 2 charge. s has a negative 2 charge They combine in 1:1 ratio The formula is therefore Fe O
What is Iron II Oxide?
Iron (II) oxide, pronounced as "iron two oxide," is an ionic compound. Ions are positively and negatively charged particles that are attracted to each other, forming a new substance. Iron (II) oxide can also be written as FeO, iron is Fe (Fe comes from the Latin ferrum) and oxide is oxygen with a name change because it is at the end of an ionic compound. Sometimes it is called ferrous oxide or iron monoxide.
What is the formula for iron oxide?
FeO is the molecular formula for iron (II) oxide. Because iron forms more than one cation, or positive ion, the Roman numeral II is used to distinguish it from the iron (III) cation.
Where is iron oxide found?
A German scientist named Friedrich Wust was the first person to synthesize the mineral. Wustite is part of a group of metal oxides called periclases. They have a 1:1 ratio of metal ion to oxygen ion. Wustite has an octahedral crystal lattice, like that of rock salt. Wustite can be found in a few places like Germany, Greenland, and India, as well as deep sea brines and inclusions in diamonds. On Mars, iron (III) oxide is responsible for the red soil, however, iron (II) oxide lends a dark contrast to the expanse.
What is the ratio of iron and oxygen?
The first is the ionic bond formula. There is a 1:1 ratio of iron ions and oxygen ions. The second formula represents what happens when elemental iron and oxygen are thrown together.
What is the most common element in the periodic table?
Oxygen is one of the most common elements. It is a gas at room temperature and is found in the p block of the periodic table. It normally forms a -2 anion, meaning it takes two electrons and forms a negatively charged ion. Oxygen is considered a nonmetal. When these two elements bond ionically (opposite charges attract) a whole new substance is formed. When iron (II) oxide comes into contact with water, a light blue/green crystal is formed. A similar phenomenon occurs when copper (II) sulfate and water come together; a beautiful blue crystal is formed. The water combines in the crystal structure of the compounds.
Why is iron added to glass?
Iron (II) oxide is added to glass to make it more heat absorbent, especially for greenhouse glass and glass windows of some buildings.
Why is it important to compare elements and compounds?
To better understand the differences between elements and the compounds they produce, the comparison of their physical and chemical properties is helpful.
How many oxygen atoms are in iron?
In contrast to the crystalline solid, in the molten state iron atoms are coordinated by predominantly 4 or 5 oxygen atoms.
What is the iron oxide in the mantle?
Within the mantle, it may be electrically conductive, which is a possible explanation for perturbations in Earth's rotation not accounted for by accepted models of the mantle's properties.
How to make a stoichiometric FeO?
Stoichiometric FeO can be prepared by heating Fe 0.95 O with metallic iron at 770 °C and 36 kbar.
What is the name of the compound that is a black powder?
Chemical compound. Iron (II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron (III) oxide (ferric oxide). Iron (II) oxide also refers ...
Is iron oxide a rock salt?
Iron (II) oxide adopts the cubic, rock salt structure, where iron atoms are octahedrally coordinated by oxygen atoms and the oxygen atoms octahedrally coordinated by iron atoms. The non-stoichiometry occurs because of the ease of oxidation of Fe II to Fe III effectively replacing a small portion of Fe II with two thirds their number of Fe III, which take up tetrahedral positions in the close packed oxide lattice.
What is iron oxide?
Iron (II) oxide is a common ingredient in many of the substances around us. It is a black powder that is used to make pigments and dyes used for pottery glazes.
Where is iron oxide found?
Iron (II) oxide is a black ionic solid that is also known as the mineral wustite, which is a black mineral. This mineral is found on Mars, but is not as common as the reddish compound iron (III) oxide, which is called hematite.
What is the formula for FeO?
This means each iron atom turns into the ion Fe +2 and each oxygen atom turns into the oxide ion O -2. Since these ions are oppositely charged they attract each other giving us the formula FeO. FeO is also known as the mineral wustite. The black rocks and dirt on Mars are iron (II) oxide.
How many electrons does an oxygen atom need to fill its outer shell?
2Fe + O 2 → 2FeO. Every oxygen atom needs two electrons to fill its outer shell. One iron atom, in this case, donates two electrons to one oxygen atom. This is where the Roman numeral II comes from after iron's symbol. This means each iron atom turns into the ion Fe +2 and each oxygen atom turns into the oxide ion O -2.
How many electrons does iron need to oxidize?
When in the presence of oxygen gas iron oxidizes. Iron (II) oxide is formed when iron is not completely oxidized. The reaction looks like: Every oxygen atom needs two electrons to fill its outer shell.
What is the Roman numeral for iron oxide?
Iron (II) oxide has the Roman numeral II indicating it is the Fe +2 ion, which has lost two electrons. When an oxygen atom takes two electrons it becomes an oxide ion with the formula O -2. The two ions combine to form iron (II) oxide with the formula FeO.
What is the color of water when it attaches to iron?
When water attaches to iron (II) oxide molecules it turns a greenish color. In this form it is also a component of green heat-absorbing glass that is commonly used in cars and windows in buildings.

Overview
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide (ferric oxide). Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron defici…
Preparation
Reactions
Structure
Occurrence in nature
Uses
See also
External links