...
| Rutherfordium | |
|---|---|
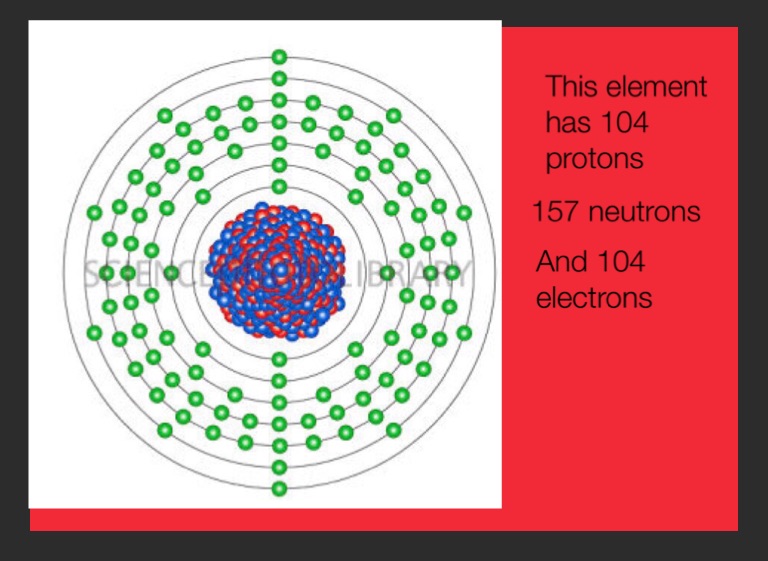
| Electrons per shell | 2, 8, 18, 32, 32, 10, 2 |
| Physical properties | |
| Phase at STP | solid (predicted) |
| Melting point | 2400 K (2100 °C, 3800 °F) (predicted) |
What are the properties of rutherfordium?
Rutherfordium is a synthetic element and little is known about its properties. It is presumed to be a solid metal. It is the first transactinide element, and is presumed to have chemical properties similar to hafnium . [See Periodic Table of the Elements]
Why is rutherfordium a transition metal?
This metal is considered a transition metal, a group of elements located in the center block of the periodic table. Lastly, rutherfordium is radioactive. This means that over time, the nuclei of this element undergo spontaneous breakdown because of their unstable properties.
Is rutherfordium a D block element?
Rutherfordium. In the periodic table of the elements, it is a d-block element and the second of the fourth-row transition elements. It is a member of the 7th period and belongs to the group 4 elements. Chemistry experiments have confirmed that rutherfordium behaves as the heavier homologue to hafnium in group 4.
What is rutherfordium (Rf)?
What is Rutherfordium? Rutherfordium is a chemical element with atomic number 104 and symbol Rf, named in honor of New Zealand physicist Ernest Rutherford. It is a synthetic element ie., an element which is not found in nature but can be prepared in the laboratory.

Is Rutherfordium a liquid?
Based on the physical state, solid, liquid or gas, elements can also be classified. Rutherfordium is a Transition metal located in the periodic table between Groups 3-12. This element is solid.
What type of element is Rutherfordium?
transuranium elementRutherfordium is a transuranium element. It is created by bombarding californium-249 with carbon-12 nuclei.
What is the state of Rutherfordium?
Rutherfordium is a synthetic chemical element. Its official chemical symbol is Rf, and its atomic number is 104, which means that a rutherfordium atom has 104 protons in its nucleus. Rutherfordium was the first super-heavy element to be discovered.
Is terbium a solid or liquid?
2007 Schools Wikipedia Selection. Related subjects: Chemical elementsGeneralPhasesolidDensity (near r.t.)8.23 g·cm−3Liquid density at m.p.7.65 g·cm−3Melting point1629 K (1356 ° C, 2473 ° F)39 more rows
Is rutherfordium a metal?
This week's element is rutherfordium, a synthetic transition metal that has the atomic symbol Rf and atomic number, 104. Its name was inspired by physicist Ernest Rutherford, who was born in New Zealand.
What are physical properties of rutherfordium?
Physical Properties of RutherfordiumAtomic Mass Average: 261.Boiling Point: 5800K.Coefficient of lineal thermal expansion/K-1: N/A.Conductivity Electrical: Thermal: 0.23 W/cmK.Description: ... Flammablity Class:Freezing Point: see melting point.Heat of Vaporization: kJ/mol.More items...
What does rutherfordium mean?
Definition of rutherfordium : a short-lived radioactive element that is produced artificially — see Chemical Elements Table.
Is rutherfordium a pure substance or mixture?
Rutherfordium is a chemical element with the symbol Rf and atomic number 104, named after New Zealand-born British physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a laboratory....RutherfordiumCAS Number53850-36-5HistoryNamingafter Ernest Rutherford27 more rows
What are 5 interesting facts about rutherfordium?
Atomic Number: 104 Atomic Symbol: Rf Atomic Weight: 265 Melting Point: Unknown Boiling Point: Unknown.Word Origin: Rutherfordium is named for scientist Ernest Rutherford.Discovery: There has been some controversy over the discovery of rutherfordium. ... Properties of rutherfordium.Sources of rutherfordium.More items...•
Can terbium be cut with a knife?
Properties of terbium The silver-gray metal, which is relatively stable in air, is malleable and can be cut with a knife.
How do you mine terbium?
Terbium can be recovered from the minerals monazite and bastnaesite by ion exchange and solvent extraction. It is also obtained from euxenite, a complex oxide containing 1% or more of terbium. The metal is usually produced commercially by reducing the anhydrous fluoride or chloride with calcium metal, under a vacuum.
Is terbium a metal?
terbium (Tb), chemical element, a rare-earth metal of the lanthanide series of the periodic table. Terbium is a moderately hard, silvery white metal that is stable in air when in pure form.
What element family is rutherfordium in?
rutherfordium (Rf), an artificially produced radioactive transuranium element in Group IVb of the periodic table, atomic number 104.
Is rutherfordium a pure substance or mixture?
Rutherfordium is a chemical element with the symbol Rf and atomic number 104, named after New Zealand-born British physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a laboratory....RutherfordiumCAS Number53850-36-5HistoryNamingafter Ernest Rutherford27 more rows
What does rutherfordium mean?
Definition of rutherfordium : a short-lived radioactive element that is produced artificially — see Chemical Elements Table.
Is Dubnium a metal nonmetal or metalloid?
The chemical element dubnium is classed as a transition metal. It was discovered in 1970 by a team of scientists led by Georgy Flerov.
Where was Rutherfordium first detected?
Rutherfordium was reportedly first detected in 1964 at the Joint Institute of Nuclear Research at Dubna (then in the Soviet Union ). Researchers there bombarded a plutonium -242 target with neon -22 ions and separated the reaction products by gradient thermochromatography after conversion to chlorides by interaction with ZrCl 4. The team identified spontaneous fission activity contained within a volatile chloride portraying eka-hafnium properties. Although a half-life was not accurately determined, later calculations indicated that the product was most likely rutherfordium-259 (abbreviated as 259 Rf in standard notation ):
Why did the Soviets name the element Kurchatovium?
As a consequence of the initial competing claims of discovery, an element naming controversy arose. Since the Soviets claimed to have first detected the new element they suggested the name kurchatovium (Ku) in honor of Igor Kurchatov (1903–1960) , former head of Soviet nuclear research.
What is the heaviest isotope produced by direct fusion?
The lightest isotopes were synthesized by direct fusion between two lighter nuclei and as decay products. The heaviest isotope produced by direct fusion is 262 Rf ; heavier isotopes have only been observed as decay products of elements with larger atomic numbers.
What is the name of the element that is derived from the Latin names for digits 1, 0, and 4?
The International Union of Pure and Applied Chemistry ( IUPAC) adopted unnilquadium (Unq) as a temporary, systematic element name, derived from the Latin names for digits 1, 0, and 4.
When was the first direct measurement of mass of a superheavy nucleus reported?
The first direct measurement of mass of a superheavy nucleus was reported in 2018 at LBNL. Mass was determined from the location of a nucleus after the transfer (the location helps determine its trajectory, which is linked to the mass-to-charge ratio of the nucleus, since the transfer was done in presence of a magnet).
When was californium synthesized?
In 1969 , researchers at the University of California, Berkeley conclusively synthesized the element by bombarding a californium -249 target with carbon-12 ions and measured the alpha decay of 257 Rf, correlated with the daughter decay of nobelium -253: 249. 98Cf.
Is Rutherfordium a homologue of Hafnium?
Rutherfordium is expected to have the electron configuration [Rn]5f 14 6d 2 7s 2 and therefore behave as the heavier homologue of hafnium in group 4 of the periodic table . It should therefore readily form a hydrated Rf 4+ ion in strong acid solution and should readily form complexes in hydrochloric acid, hydrobromic or hydrofluoric acid solutions.
What is Rutherfordium named after?
Rutherfordium is a synthetic element, named in honour of eminent scientist of Britain, Ernest Rutherford. It is a synthetic element that means it is not available in nature. You can prepare it in the laboratory.
How was Rutherfordium Discovered?
There are some controversies involved with who discovered rutherfordium. A group of scientists in Dubna, Russia first reported about its discovery in the year 1964. Russian scientists in the Joint Institute of Nuclear Research at Dubna, Russia, U.S.S.R. declared the discovery of element 104. They named the element as kurchatovium, with the symbol Ku following the name of a Soviet Nuclear Physicist Igor Kurchatov. An isotope, 260Rf was produced that had 0.3 seconds of half-life. Then they changed the half-life to 0.15 seconds.
What is the half life of 104?
In Dubna, the Soviet researchers bombarded Plutonium 242 along with the ions of neon 22. They made a declaration of obtaining an isotope of element 104 whose mass number is 260 and half-life of 0.3 seconds. Then they performed numerous experiments on the isotope newly discovered and claimed that it behaved the same way as they predicted for it. Next time, when they used a more advanced method for half-life measurement, it came out different from the earlier one. They found the half-life of the element is 0.1 seconds, not 0.3 seconds as they reported earlier. The mismatch in results creates doubt on the chemical experiments.
How long does Rutherfordium decay?
12 isotopes of Rutherfordium are recognised along with their half-lives. The most stable one among the 12 isotopes is 265Rf having a half-life of about 13 hours and decays through spontaneous fission.
What is the mass number of Curium 248?
This isotope has a mass number of 261and half-life 70 seconds .
What is the name of the isotopes that are produced by decaying?
alpha particles and detect the products produced on decaying, which are known as nobelium isotopes.
When did the Dubna gang regenerate the isotopes?
In the year of 1969 , a team of American researchers at the Lawrence Radiation Laboratory of the University of California, Berkeley attempted to regenerate the result of the isotope identified by the Dubna gang. They announced that they have been able to identify the isotopes of the element which are different from the U.S.S.R. lab. This time, they had been able to produce four isotopes of Rutherfordium by following a different method. The controversy is, whether they produced 260Rf or not, is not confirmed in the report.
What is Rutherfordium?
Rutherfordium is a synthetic chemical element that has an atomic number of 104 and is represented by the symbol “Rf”, named after the physicist Ernest Rutherford from New Zealand. It is a synthetic element as it cannot exist naturally and can only be prepared in the laboratory. It is often created by bombarding Carbon-12 nuclei on Californium-249.
Rutherfordium: Discovery
The discovery of Rutherfordium is said to be controversial as there is confusion between who discovered rutherfordium. The discovery was first reported in 1964 by a group of scientists in Dubna, Russia. The Joint Institute of Nuclear Research at Dubna, Russia, U.S.S.R.
Things To Remember
Rutherfordium is an element named in honour of Ernest Rutherford, the eminent scientist of Britain. It is not available in nature and hence is called a synthetic element.
How is Rutherfordium made?
In order to make synthetic elements, scientists use a process called artificially induced radioactivity. With this method, scientists bombard elements together, hoping that protons, electrons, and neutrons will stick together and form new elements. Ernest Rutherford was the one who introduced this method to science. Since then, more and more synthetic elements are being made within the lab.
Why was the element Rutherfordium named Rutherfordium?
Because both Russia and the United States had discovered this element, it became very difficult for the IUPAC, or the International Unions of Pure and Applied Chemistry, to settle on a name. Both countries had been justified when it came to the discoveries. However, there was already a rivalry between the two countries, so the name was never decided until 1990's. The IUPAC decided to name the element rutherfordium, which is a name giving honor to Ernest Rutherford, who had discovered the method for making this element.
What element did Georgy Flerov discover?
In 1964, Georgy Flerov was working with a team of scientists within the Russian Joint Institute for Nuclear Research. They were conducting nuclear research by bombarding elements together. As they combined plutonium with neon, they produced an isotope of element 104. At the time, element 104 didn't exist, and by 1966 they were able to confirm that they had in fact discovered a new element.
What is the atomic mass of Rutherfordium?
Rutherfordium has an atomic number of 104 and an atomic mass of 261 AMU. Atomic number is the number of protons in the nucleus of the element. As explained in the history of this element, rutherfordium has several isotopes or different forms of the element with different numbers of neutrons. When discovered, Russia was able to make Rf-259, while scientists from Berkeley, California were able to make the isotopes Rf-257, Rf-258, and Rf-260.
Where was the element 104 discovered?
At the same time that Flerov's team discovered this new element, Albert Ghiorso was working with his team of scientists at the Lawrence Berkeley laboratory in Berkeley, California. They were conducting the same research and found not just one isotope of element 104, but three. They confirmed their discovery of the new element in 1969.
Was Rutherfordium made in two different labs?
Behind laboratory doors, rutherfordium was just being discovered and made within two different labs, which would cause a conflict on its own. Here in this lesson, let us have a look at the history, facts, and uses of rutherfordium.
Is Rutherfordium a solid?
Rutherfordium is a metal that is solid at room temperature. Because only a few atoms of this element have been made, it is difficult to say what this metal looks like. This metal is considered a transition metal, a group of elements located in the center block of the periodic table. Lastly, rutherfordium is radioactive. This means that over time, the nuclei of this element undergo spontaneous breakdown because of their unstable properties.
What is Rutherfordium?from thoughtco.com
Properties of Rutherfordium. Rutherfordium is a d-block element, member of the 7th period and belongs to group 4 elements in the periodic table. It is a kind of trans-uranium and radioactive element which cannot be found in nature. It does not have any stable or naturally occurring isotopes.
What is the atomic number of Rutherfordium?from byjus.com
Rutherfordium is a chemical element with symbol Rf and atomic number 104. Classified as a transition metal, Rutherfordium is a solid at room temperature. H.
How long does rutherfordium last?from thoughtco.com
Isotopes: All of the isotopes of rutherfordium are radioactive and synthetic. The most stable isotope, Rf-267, has a half-life around 1.3 hours.
What was the name of the lab that discovered that plutonium decayed?from pubchem.ncbi.nlm.nih.gov
Jefferson Lab, U.S. Department of Energy. In 1964, workers at the Joint Nuclear Research Institute at Dubna (U.S.S.R.) bombarded plutonium with accelerated 113 to 115 MeV neon ions. By measuring fission tracks in a special glass with a microscope, they detected an isotope that decays by spontaneous fission.
How is element 259 104 formed?from pubchem.ncbi.nlm.nih.gov
Element 259 104 is formed by the merging of a 13 C nuclei with 249 Cf, followed by emission of three neutrons. This isotope has a half-life of 3 to 4 s, and decays by emitting an alpha particle into 255 No, which has a half-life of 185 s.
What is the half life of 257 104?from pubchem.ncbi.nlm.nih.gov
The combination of 12 C with 249 Cf followed by instant emission of four neutrons produced Element 257 104. This isotope has a half-life of 4 to 5 s, decaying by emitting an alpha particle into 253 No, with a half-life of 105 s.
Which element was isolated by the Dubna team?from softschools.com
In their attempts to reprove their claims on the discovery of the element, the Dubna team isolated the gaseous phase of rutherfordium.
What is the atomic number of Rutherfordium?
Rutherfordium is a chemical element with atomic number 104 and symbol Rf, named in honor of New Zealand physicist Ernest Rutherford. It is a synthetic element ie., an element which is not found in nature but can be prepared in the laboratory. It can be created by bombarding Californium -249 with a Carbon-12 nuclei.
Is Rutherfordium a radioactive element?
It is a kind of trans-uranium and radioactive element which cannot be found in nature. It does not have any stable or naturally occurring isotopes.
How many protons does Rutherfordium have?
Rutherfordium is a chemical element with atomic number 104 which means there are 104 protons and 104 electrons in the atomic structure. The chemical symbol for Rutherfordium is Rf.
Which atom is the least electronegative?
The most electronegative atom, fluorine, is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7.

Overview
Predicted properties
Very few properties of rutherfordium or its compounds have been measured; this is due to its extremely limited and expensive production and the fact that rutherfordium (and its parents) decays very quickly. A few singular chemistry-related properties have been measured, but properties of rutherfordium metal remain unknown and only predictions are available.
Rutherfordium is the first transactinide element and the second member of the 6d series of transit…
Introduction
The heaviest atomic nuclei are created in nuclear reactions that combine two other nuclei of unequal size into one; roughly, the more unequal the two nuclei in terms of mass, the greater the possibility that the two react. The material made of the heavier nuclei is made into a target, which is then bombarded by the beam of lighter nuclei. Two nuclei can only fuse into one if they approach e…
History
Rutherfordium was reportedly first detected in 1964 at Joint Institute for Nuclear Research at Dubna (Soviet Union at the time). Researchers there bombarded a plutonium-242 target with neon-22 ions and separated the reaction products by gradient thermochromatography after conversion to chlorides by interaction with ZrCl4. The team identified spontaneous fission activity contained within a volatil…
Isotopes
Rutherfordium has no stable or naturally occurring isotopes. Several radioactive isotopes have been synthesized in the laboratory, either by fusing two atoms or by observing the decay of heavier elements. Sixteen different isotopes have been reported with atomic masses from 253 to 270 (with the exceptions of 264 and 269). Most of these decay predominantly through spontaneous fission pathways.
Experimental chemistry
Early work on the study of the chemistry of rutherfordium focused on gas thermochromatography and measurement of relative deposition temperature adsorption curves. The initial work was carried out at Dubna in an attempt to reaffirm their discovery of the element. Recent work is more reliable regarding the identification of the parent rutherfordium radioisotopes. The isotope Rf has been used for these studies, though the long-lived isotope Rf (produced in the decay chain of Lv…
Bibliography
• Audi, G.; Kondev, F. G.; Wang, M.; et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
• Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
External links
• Media related to Rutherfordium at Wikimedia Commons
• Rutherfordium at The Periodic Table of Videos (University of Nottingham)
• WebElements.com – Rutherfordium