What are the characteristics of metals and non-metals?
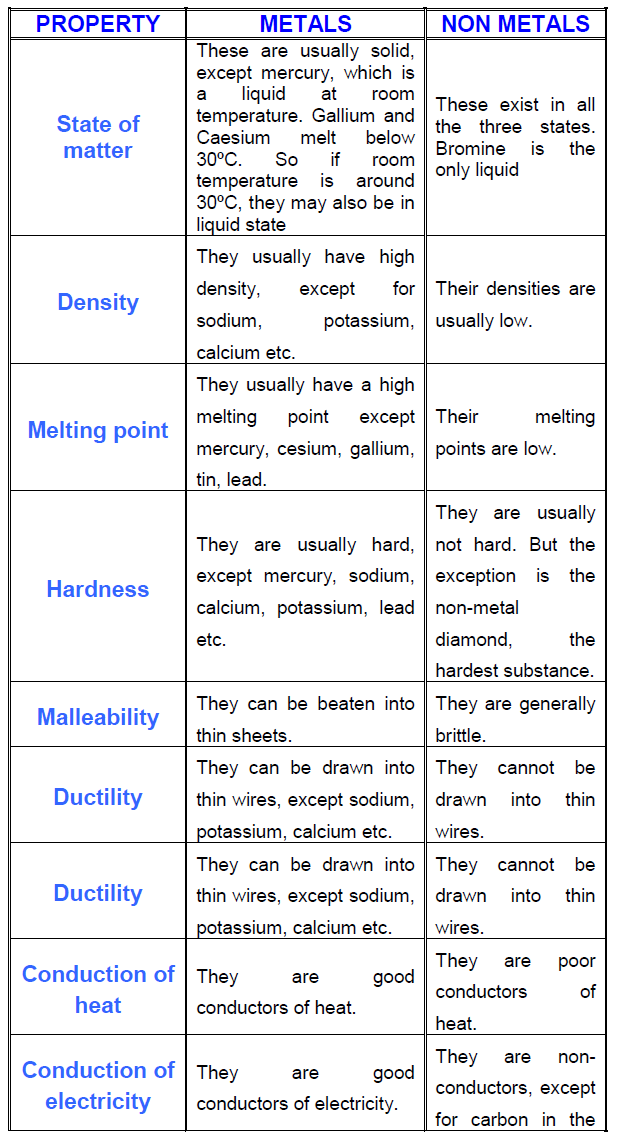
Characteristics of metals and non-metals Non-Metals are not malleable. Non-Metals are not ductile. They are bad conductor of heat and electricity. They are not lustrous or shiny.
What properties do all non-metals have in common?
Properties commonly seen in nonmetals are:
- for ionic/covalent bonds
- brittle and nonmalleable
- low melting/boiling points
- High ionization energy and electronegativity
- poor conductors of heat and electricity
How are nonmetals different from metals?
Properties of Non metals
- The atoms of non-metals tend to be smaller than those of metals. ...
- Non-metals exhibit very low electrical conductivities. ...
- Non-metals have high electronegativities. ...
- Non-metals have high electronegativities. ...
- Under normal conditions of temperature and pressure, some non-metals are found as gases, some found as solids and one is found as liquid. ...
What are the characteristic of metals and non metals?
Metals: Non-Metals; Hardness: Metals are generally hard solids except zinc which is brittle: Non-Metals are brittle in nature and easily break down. Malleability: Metals are highly malleable. Non-metals are not malleable. Conductivity of Electricity: Metals are very good conductors of electricity: Non-metals are generally poor conductors of electricity.

What are properties of metals?
Metals are lustrous, malleable, ductile, good conductors of heat and electricity. Other properties include: State: Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (Gallium is liquid on hot days).
What are 10 properties of metals?
Physical PropertiesMetals occur in the solid state. All metals are solid except with an exception for mercury which is in liquid state in its natural form.Metals are malleable in nature. They can be beaten into thin sheets. ... Metals are ductile. ... Metals conduct heat and electricity.
What are the 7 properties of non-metals?
Here is a summary of the properties of the nonmetals.High ionization energies.High electronegativities.Poor thermal conductors.Poor electrical conductors.Brittle solids—not malleable or ductile.Little or no metallic luster.Gain electrons easily.Dull, not metallic-shiny, although they may be colorful.More items...•
What are the properties of metal answer?
Five physical properties of metals are:Metals are malleable and ductile.Metals are good conductors of heat and electricity.Metals are lustrous (shiny) and can be polished.Metals are solids at room temperature (except mercury, which is liquid).Metals are tough and strong.
What are the 20 properties of metals?
Properties of MetalsMetals can be hammered into thin sheets. It means they possess the property of malleability.Metals are ductile. ... Metals are a good conductor of heat and electricity.Metals are lustrous which means they have a shiny appearance.Metals have high tensile strength. ... Metals are sonorous. ... Metals are hard.
What are the 18 nonmetals?
In the above table nonmetal elements are H,He,C,N,O,F,Ne,P,S,Cl,Ar,Se,Br,Kr,I,Xe,At and Rn.
What are the 20 nonmetals?
Metals in the first twenty elements are Lithium, Beryllium, Sodium, magnesium, Aluminum, Potassium, and calcium. Now the non-metals in the first twenty elements are Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon.
What are the 10 examples of metals?
Examples of metals are aluminium, copper, iron, tin, gold, lead, silver, titanium, uranium, and zinc. Well-known alloys include bronze and steel. The study of metals is called metallurgy.
What are the 5 non-metals?
Hydrogen, carbon, nitrogen, oxygen, phosphorus, arsenic and selenium are the non-metallic elements in the periodic table.
What is non metal short answer?
What are non-metals? Answer: Substances which are soft and dull, i.e., non-lustrous, non-sonorous, non-ductile, non-malleable and poor conductor of heat and electricity are called non-metals. For example, oxygen, hydrogen, sulphur, etc.
What are the properties of metals Class 6?
Properties of Metals:They are lustrous, i.e. they shine.They are malleable, i.e. they can be drawn into sheets.They are ductile, i.e. they can be made into wires.They are good conductors of heat and electricity.They have high density.They have high melting and boiling points.
What are the 10 properties of non metals?
Physical Properties of NonmetalsNonmetals have high ionization energies.They have high electronegativities.Nonmetals are insulators which means that they're poor conductors of electricity.They are dull, they do not have lustre like metals.Nonmetals are poor conductors of heat. ... They are very weak and brittle.More items...
Q.1. What are the 5 physical properties of metals?
Ans: The five physical properties of metals include malleability, ductility, lustre, sonority, thermal conductivity and density.
Q.2. Is iodine a metal?
Ans: No, iodine is a non-metal.
Q.3. What are the physical and chemical properties of metals?
Ans: The physical properties of the metals include malleability, ductility, lustre, sonority, density, thermal and electrical conductivity, melting...
Q.4. What are the 4 physical properties of non-metals?
Ans: Non-metals are entirely different from metals. They are non-malleable, non-ductile, non-lustrous and poor conductors of heat and electricity.
Q.5. Is mercury a solid?
Ans: No, mercury is a liquid at room temperature.
What are the physical properties of metals and non-metals?
We are now familiar with the physical properties of metals and non-metals. Metals are the elements that conduct heat and electricity and are malleable and ductile. They are also lustrous, hard, strong, heavy and sonorous too. Non-metals are the elements that do not conduct electricity and heat and are neither malleable nor ductile. They are brittle, non-lustrous, soft, brittle and non-sonorous. By looking at these properties explained in the article, we can now differentiate metals and non-metals easily.
How many electrons are in metals?
Metals generally have 1, 2 or 3 electrons in their outermost shell. For example, the electronic configurations of some metals are given below:
What is malleable material?
The name for this property is malleability. The majority of metals are malleable by nature. Gold and silver are the most malleable metals among these.
What is the property of a material to shine when light is incident on it?
The property of a material to shine when light is incident on it is known as lustre. Generally, metals are lustrous in nature, and they can be polished too.
Which metal is the best conductor of heat and electricity?
Metals are generally good conductors of heat and electricity. Silver is the best conductor of heat and electricity and lead is the poorest. The kitchen utensils found in our home are made of zinc, copper and aluminum. This is because these metals are good conductors of heat.
Is potassium a hard metal?
Metals are generally very hard. They cannot be cut or compressed so easily. However, metals like sodium and potassium are soft so that they can be cut using a knife. They are hence called soft metals.
Is mercury a solid or liquid?
Metals are generally solids at room temperature. An exception is there that; the metal mercury exists as a liquid at room temperature.
What is a Metal and Non-Metal?
Metals are bulk of elements present in the periodic table, it is shiny in nature, conduct heat as well as electricity, and malleable and ductile. For example iron, copper, gold and silver are Metals. On the other hand, Non-Metals lie on the left side of the periodic table. Non-Metals does not show any metallic property like it does not conduct heat and electricity, neither malleable nor ductile in nature. Oxygen, Carbon are some examples of Non-Metals. Also, non-metals majorly exist in the gaseous state while metals present in the solid-state.
What does it mean when a metal has low electronegativity?
Have low electronegativities: Electronegativity is referred to the ability of the atom to attract electrons to form a chemical bond. So high electronegativity means it will attract electrons very easily, and low electronegativity means less ability to attract electrons. Thus, metals have low electronegativity.
Why is gold shiny?
This is due to the Lustrous property of metals which makes gold and silver shiny and attractable.
What is malleable metal?
Malleable and Ductile: Metals are malleable means they can be beaten into a sheet by hammer or rolling. Also, metals are ductile in nature i.e. they can be easily stretched into a wire when they get pulled. Gold is the most ductile metal that can stretch up to 1 km with the use of 1 gm of gold only.
What does lustrous mean in metals?
Lustrous: Metals are lustrous in nature, but what does it mean? Lustrous means shiny surface for example gold and silvery have metallic lustre thus they are used for making jewellery. Also, utensils made of metals have a metallic lustre.
What does it mean when a non-metal is brittle?
Brittle: Non-Metals are very much brittle in nature means they can break if they got stretched or beaten, that means non-metal neither malleable nor ductile in nature.
What are the two types of materials?
The elements further divided into two material called Metals and Non-Metals. Just look around yourself you are surrounded by the elements, now the question is can you differentiate what is metal or non-metal? Is copper wire or aluminium window is metal or non-metal? So in science, there are some properties mainly physical and chemical properties which help us to distinguish between the two. For example suppose there is cow and dog, although both are animals, still, we can differentiate them easily by just seeing them. Both look completely different to each other, and we can easily tell that look this is a dog and that is a cow. Before differentiating between two let’s first understand what actually they are?
Where are metals and nonmetals on the periodic table?
Properties of metal and non-metal elements. Metals are placed on the left-hand side of the periodic table, and non-metals on the right.
What are some examples of elements that have properties that are not typical?
Some elements have properties that are not typical. For example: mercury (a metal) has a low melting point and exists as a liquid at room temperature. graphite, a form of carbon (a non-metal), has a high boiling point and is also a good conductor of electricity. A substance with a high density means it has a high mass for its size.
Which is more likely to corrode: metals or non-metals?
Metals are also more likely to corrode than non-metals. This means that non-metals are less likely to react with water or acids than metals are.
What are some examples of chemical properties?
The most common chemical property is the type of oxide that the element forms. Metals form oxides that are basic, but non-metals form oxides that are acidic. For example, sulfur and carbon are both non-metals. They react with oxygen to form sulfur dioxide and carbon dioxide.
What are the properties of metals, metalloids and nonmetals?
Periods (1–7, ...) Blocks (s, p, d, f, ...) The chemical elements can be broadly divided into metals, metalloids and nonmetals according to their shared physical and chemical properties . All metals have a shiny appearance (at least when freshly polished); are good conductors of heat and electricity;
What are the two types of metals on the periodic table?
From left to right in the periodic table, these categories include the highly reactive alkali metals; the less reactive alkaline earth metals, lanthanides and radioactive actinides; the archetypal transition metals, and the physically and chemically weak post-transition metals.
What are the elements that are a good conductor of heat and electricity?
v. t. e. The chemical elements can be broadly divided into metals, metalloids and nonmetals according to their shared physical and chemical properties. All metals have a shiny appearance (at least when freshly polished); are good conductors of heat and electricity; form alloys with other metals; and have at least one basic oxide.
What is a metaloid?
Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms, and have amphoteric or weakly acidic oxides. Typical nonmetals have a dull, coloured or colourless appearance; are brittle when solid; are poor conductors of heat and electricity; and have acidic oxides.
How many atoms are in a manganese crystal?
Well-behaved metals have crystal structures featuring unit cells with up to four atoms. Manganese has a complex crystal structure with a 58-atom unit cell, effectively four different atomic radii, and four different coordination numbers (10, 11, 12 and 16).
Which metal has the highest ionization energy?
The only metal having an ionisation energy higher than some nonmetals ( sulfur and selenium) is mercury. Mer cury and its compounds have a reputation for toxicity but on a scale of 1 to 10, dimethylmercury ( (CH 3) 2 Hg) (abbr. DMM), a volatile colourless liquid, has been described as a 15.
Which element has the longest half life?
Bismuth has the longest half-life of any naturally occurring element; its only primordial isotope, bismuth-209, was found in 2003 to be slightly radioactive, decaying via alpha decay with a half-life more than a billion times the estimated age of the universe. Prior to this discovery, bismuth-209 was thought to be the heaviest naturally occurring stable isotope; this distinction now belongs to lead-208.
What are non metals?
Non-metals are elements that form negative ions by gaining electrons during chemical reactions. Thus, they are electronegative elements with high ionization energies. In general, non-metals are brittle, dull, and poor conductors of heat and electricity.
Which is more dense, metals or nonmetals?
Metals exhibit a wide range of densities, but generally are more dense than nonmetals. Tungsten, platinum, osmium, gold and iridium are extremely dense.
What are the properties of semi-metals?
Semi-metals, also known as metalloids, have properties of both metals and non-metals. Metalloids can be shiny or dull. They are typically semi-conductors. Semi-conductors are capable of conducting electricity better than insulator, but not as well as conductors.
What is an element?
Introduction to Metals, Semi-metals, and Non-metals. An element is a substance that cannot be broken down into any other substance. In other words, an element is the simplest form of matter. Elements are further classified into metals, non-metals, and semi-metals.
Is metal a conductor of heat?
Furthermore, they are ductile, malleable, and lustrous. Metals are also good conductors of heat and electricity.
Do metals form ionic bonds?
Metals generally form ion ic bonds with nonmetals, but there are exceptions. Most metals form at least one basic oxide, although some are amphoteric. Metals exhibit a wide range of reactivity. Special groups of metals include the noble metals Ru, Rh, Pd, Pt, Au, Os, Ir, Ag and the refractory metals Nb, Mo, Ta, W and Re.
Is a semi-conductors a conductor?
They are brittle, and are typically semi-conductors. Semi-conductors are capable of conducting electricity better than insulator, but not as well as conductors.
How many electrons are in a non-metal?
Following are some of the chemical properties of Non-metals: Electronic configuration- Non-metals have 5, 6 or 7 electrons in their valence shell in their electronic configuration. [Exception: Hydrogen which has only 1 electron but still a non-metal] Valency- Non-metals have -1, -2 or -3 valencies.
What is the state of metals?
These properties are shown below: State- Metals are hard and crystalline solids (except mercury – which is a liquid) Metallic Lustre- Metals in their pure state shine. This property is called metallic lustre. In other words, they shine when they are polished.
What is the reaction of metals to oxygen?
Reaction with oxygen and the nature of the oxide- Metals reacts with oxygen to form their respective oxides. These oxides are basic in nature. There are some oxides that are also soluble in water. They are called alkali. Example: Metal +Oxygen → Metal Oxide (Heat) (basic) 4Na + O2 → 2Na2O. 4Cu + O2 → 2Cu2O.
How many electrons are in the outermost shell of a spherical sphere?
They generally have 3 to 6 electrons in their valence shell (or outermost shell).
What are the three categories of substances?
of materials. They are called elements. Now, these elements are also classified into three categories – Metals, Non-Metals and Metalloids.
Which metal has the highest density?
Density- Metals have a high density (except sodium, potassium and lithium) Hardness- Almost all the metals are hard solids except sodium and potassium which can be cut through a knife. Melting and Boiling Points- Metals usually have high melting and boiling points.
Is gold a malleable metal?
Gold, silver, copper are highly malleable. (Exception – Zinc is brittle) Sonority- Metals when struck by a hard object they produce a sound. This property is called sonority. Thermal and electrical conductivity- Metals are good conductors of heat and electricity.
What is the property of metals that make a sound when struck by another object?
Sonorousness: Metals make ringing or vibrating sound when struck with another hard object, this property is called Sonorousness.
What state are non-metals found in?
State: Most of the Non-metals are found in a liquid or gaseous states, some Non-Metals are in solid-state.
What is malleable metal?
Malleable: Malleability refers to the property of metals by which they can be beaten into thin sheets.
Where are metals found?
Metals are natural compounds of the earth’s crust, where they are usually found in the form of ores, which is composed of both one and many other materials. And they are naturally present in rocks washed away by surface water and groundwater and in the atmosphere.
Which side of the periodic table are metals arranged?
An element that lacks metal properties and is capable of forming anions, acid oxides, acids, and stable hydrogen compounds. Metals are arranged Right-side of the periodic table.
Is graphite a poor conductor?
Poor conductors: Conductivity is the property of allowing electricity or heat to pass through them, but Non-Metals are poor conductors except graphite
Is metal a good conductor of heat?
Conductivity: Metals are good conductors of heat and electricity, they allow electricity and heat to pass through them
What is a nonmetal?
in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. A nonmetal is simply an element that does not display the properties of a metal.
How are nonmetals separated from metals?
Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The halogens and noble gases are nonmetals, but the nonmetal element group usually consists of the following elements: hydrogen. carbon. nitrogen. oxygen.
What are the properties of a spherical sphere?
Summary of Common Properties 1 High ionization energies 2 High electronegativities 3 Poor thermal conductors 4 Poor electrical conductors 5 Brittle solids—not malleable or ductile 6 Little or no metallic luster 7 Gain electrons easily 8 Dull, not metallic-shiny, although they may be colorful 9 Lower melting points and boiling point than the metals
Is 118 a metal or a liquid?
element 118 (oganesson). This element is predicted to be a liquid but is still a nonmetal.
Is nonmetal a metal?
It doesn't look metallic, can't be made into a wire, pounded into shape or bent, doesn't conduct heat or electricity well, and doesn't have a high melting or boiling point. The nonmetals are in a minority on the periodic table, mostly located on the right-hand side of the periodic table.
