Atomic Number of Gold Gold is a chemical element with atomic number 79
Gold
Gold is a chemical element with the symbol Au and atomic number 79, making it one of the higher atomic number elements that occur naturally. In its purest form, it is a bright, slightly reddish yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal and a grou…
How many atoms of gold are in 1 gram of gold?
SO 1.0g of gold must be around: atomic weight of Au (gold)=196.97 Avogadro’s number=6.022X10^23 (1.0 g)/ (196.97 g/mol) = 0.005076915 mol 0.005076915 mol x 6.022X10^23 = 3.057318373 x 10^21 ATOMS of Gold (Au) that have 30,573,183,730,000,000,000 = 30.573 Billion-Billion Atoms of Gold in 1 gram.
How would you create a gold atom?
Gold is the chemical element with 79 protons in each atomic nucleus. Every atom containing 79 protons is a gold atom, and all gold atoms behave the same chemically. In principle, we can therefore create gold by simply assembling 79 protons (and enough neutrons to make the nucleus stable).
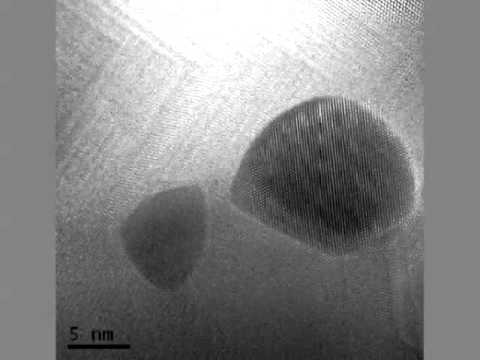
What does the atom of gold look like?
Gold. Gold is the third element in the eleventh column of the periodic table. It is classified as a transition metal. Gold atoms have 79 electrons and 79 protons with 118 neutrons in the most abundant isotope. Under standard conditions gold is a shiny yellow metal. It is very dense and heavy, but also fairly soft.
How big is an atom of gold?
The numbers of particles in an atom can be calculated from its atomic number and mass number. Atoms are so small that it is easier to write their size using standard form. An atom of gold has a diameter of 0.000,000,000,144 m. To write this number in standard form:

What type of atom is gold made of?
Gold is a chemical element with symbol Au and atomic number 79. Classified as a transition metal, Gold is a solid at room temperature.
How many atoms are in a gold atom?
Answer and Explanation: There are 6.022 X 1023 atoms in one mole of gold. A mole will have the same number of units whether we are trying to count atoms or molecules.
What is gold atom or molecule?
Gold is an atom with 79 protons in the nucleus. All neutral atoms (no charge) have the exact same number of electrons as they have protons. Neutrons vary but will typically be the same as the number of protons (smaller atoms) or 1.5 times the number of protons (larger atoms).
Is gold example of atom?
Gold is a chemical element with the symbol Au (from Latin: aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally.
How do you find atoms in gold?
Explanation: Calculate the moles of gold by dividing the given mass by its molar mass, 196.966569 g/mol (atomic weight on periodic table in g/mol). Multiply the calculated mol Au times 6.022×1023atoms1mol .
How much is an atom?
Atom can be purchased for $139. Additionally you can purchase Atom through our payment plan program, which splits your payments into 16 total payments of $9 each paid every 30 days.
Is gold a single atom?
Gold is the chemical element with 79 protons in each atomic nucleus. Every atom containing 79 protons is a gold atom, and all gold atoms behave the same chemically. In principle, we can therefore create gold by simply assembling 79 protons (and enough neutrons to make the nucleus stable).
What is the molecules of gold?
GoldPubChem CID23985Chemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaAuSynonyms7440-57-5 Au Gold Colloidal gold Gold powder More...Molecular Weight196.966573 more rows
How many electrons are in a gold atom?
79NameGoldNumber of Protons79Number of Neutrons118Number of Electrons79Melting Point1064.43° C9 more rows
How many atoms are in an element?
An atom is an element. The two words are synonymous, so if you're looking for the number of atoms in an element, the answer is always one, and only one.
What is element and atom?
An element is the simplest form of a substance. Generally, it cannot be simplified or broken down further into smaller particles. An atom is the part of an element. A particular element is composed of only one type of atom. Atoms are further composed of subatomic particles called electrons, protons and neutrons.
What are atoms made of?
Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom's nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom's nucleus.
How big is a gold atom?
How big is an atom of gold? The approximate atomic radius of gold is 0.1441 nanometers or 0.1441 x 10-9 meters (i.e. 0.1441 millionth of a milimeter!)
How many atoms are in 1g of gold?
This is an Expert-Verified Answer Atomic weight of the Gold (Au) is = 196.97 (197g apprx.) That means 6.022X10^23 atoms weigh 196.97 g.
How many neutrons are in a gold atom?
79Gold / Atomic number
How many electrons does a gold atom have?
79NameGoldNumber of Protons79Number of Neutrons118Number of Electrons79Melting Point1064.43° C9 more rows
Overview
Characteristics
Gold is the most malleable of all metals. It can be drawn into a wire of single-atom width, and then stretched considerably before it breaks. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet (28 …
Chemistry
Origin
Occurrence
History
Production
Monetary use