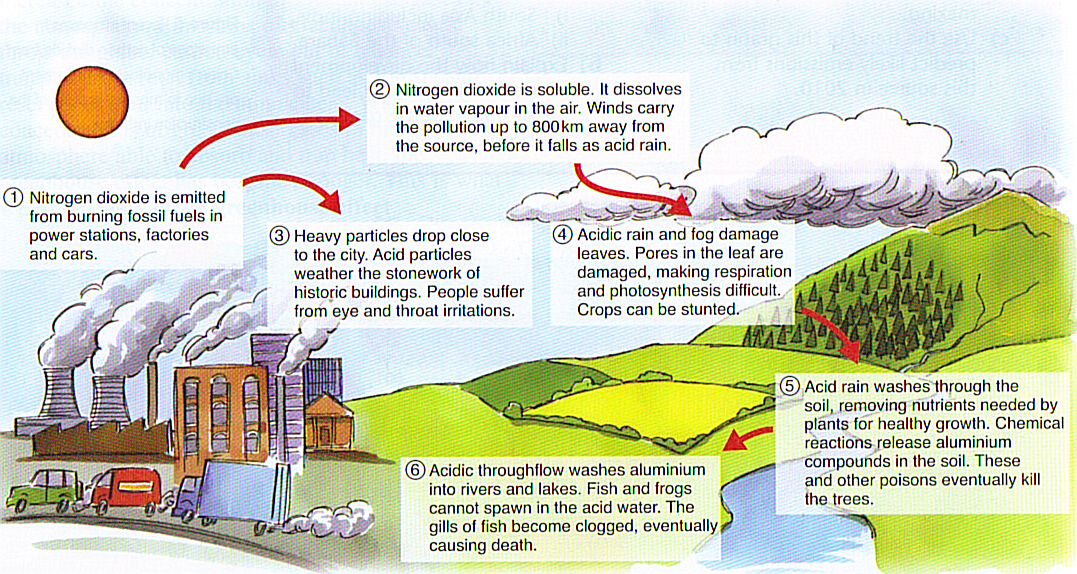
Man-made or Anthropogenic Causes of Acid Rain
- Combustion of coal and oil As stated earlier, the principal emissions accountable for acidic depositions in the atmosphere are oxides of nitrogen (NOx) and sulfur dioxide (SO2). ...
- Power plants and manufacturing industries Contemporary power plants use fuel to generate energy. ...
- Automobiles and other vehicles
What are 3 ways to reduce acid rain?
What are three ways to reduce acid rain? Alternative energy sources should be used, such as solar and wind power. Renewable sources of energy are helping to reduce acid rain, as they produce much fewer emissions. There are other electricity sources as well, such as nuclear power, hydropower, and geothermal energy.
What are two harmful effects of acid rain?
What are two harmful effects of acid rain? Dead or dying trees are a common sight in areas effected by acid rain. Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
What causes acid rain and how can we prevent it?
Acid rain occurs when sulfur dioxide and nitrogen oxides mix with the molecules in the atmosphere and increase the acidity of precipitation. Though called acid rain, it can also be snow, sleet, or even just dry particles in the air. As we work to reduce our fossil fuel emissions, we can reduce the effects of acid rain.
What is primarily causes acid rain?
- Question 1 SURVEY 30 seconds Q. ...
- Question 2 SURVEY 30 seconds Q. ...
- Question 3 SURVEY 30 seconds Q. ...
- Question 4 SURVEY 30 seconds Q. ...
- Question 5 SURVEY 30 seconds Q. ...
- Question 6 SURVEY 30 seconds Q. ...
- Question 7 SURVEY 30 seconds Q. ...
- Question 8 SURVEY 45 seconds Q. ...
- Question 9 SURVEY 30 seconds Q. ...
- Question 10 SURVEY 30 seconds Q. ...
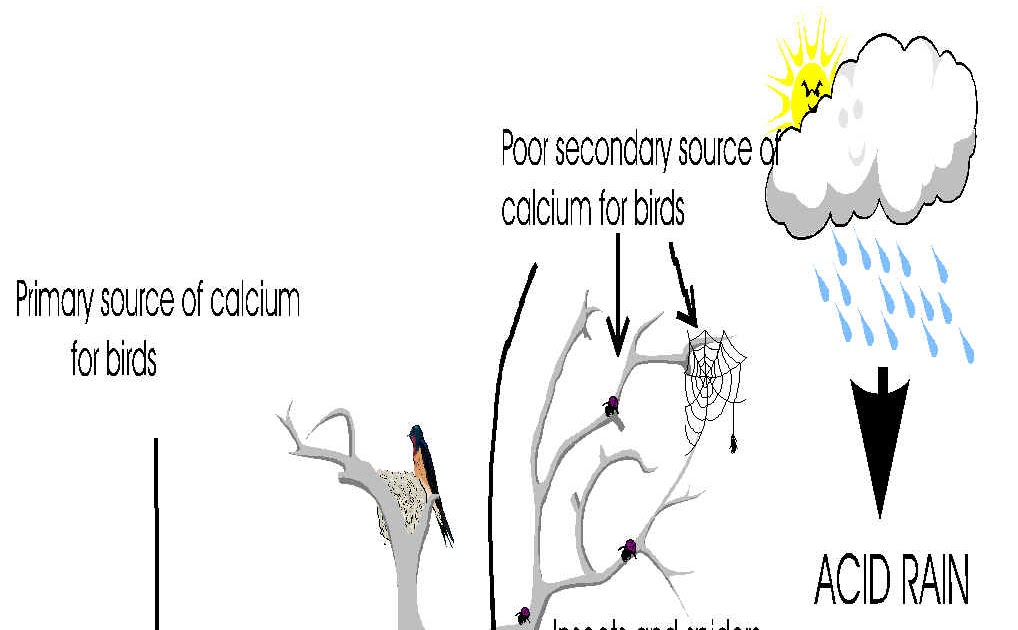
What are 5 causes of acid rain?
Man-made or Anthropogenic Causes of Acid RainCombustion of coal and oil.Power plants and manufacturing industries.Automobiles and other vehicles.Volcanic eruptions: The main natural causal agent for acid rain is volcanic emissions.
What are three causes of acid rain?
Causes of acid rain Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants, factories, and automobiles.
What causes acid rain and how can it be prevented?
Because nitrogen oxides are created in the process of burning coal and other fossil fuels, some power plants are changing the way they burn coal. A great way to reduce acid rain is to produce energy without using fossil fuels. Instead, people can use renewable energy sources, such as solar and wind power.
What are two ways that cause acid rain?
That is a condition that will dissolve rocks," said study team member John Valley. Sulfur dioxide (SO2) and nitrogen oxides (NOx) released into the air by fossil-fuel power plants, vehicles and oil refineries are the biggest cause of acid rain today, according to the Environmental Protection Agency (EPA).
What are the causes and effects of acid rain on environment?
Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
Which gas is caused acid rain?
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
What is the cause of acid rain How is it harmful to the environment?
Acid Rain Harms Forests Acid rain that seeps into the ground can dissolve nutrients, such as magnesium and calcium, that trees need to be healthy. Acid rain also causes aluminum to be released into the soil, which makes it difficult for trees to take up water.
What is acid rain and its effects?
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
What is acid rain Short answer?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
Is acid rain caused by air pollution?
Acid rain is one of the consequences of air pollution. Gases produced from the burning of fuels react with the oxygen in the air and water vapour, transforming into acids that fall onto the earth's surface as rain.
What is acid rain for kids?
Acid rain is formed when pollutants called oxides of sulfur and nitrogen, contained in power plant smoke, factory smoke, and car exhaust, react with the moisture in the atmosphere. Dry deposition, such as soot and ash, sleet, hail, snow, smog and low level ozone are forms that acid rain can take, despite its name.
What is acid rain and how is it caused?
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. The...
What are the effects of acid rain?
The ecological consequences of acid rain are seen most strongly in marine habitats, such as streams, lakes and marshes where fish and other wildlif...
What will happen if we don’t stop acid rain?
Sulphur dioxide and nitrogen oxide are the principal chemicals for acid rain. It can also influence humans since the acid goes into fruits, vegetab...
What is acid rain? What are its harmful effects?
It has been shown that acid rain has detrimental effects on trees, freshwaters and soils, destroys insects and aquatic life-forms, causes paint to...
What are three ways to reduce acid rain?
Alternative energy sources should be used, such as solar and wind power. Renewable sources of energy are helping to reduce acid rain, as they produ...
How does acid rain affect plants?
Acid rain can affect the health of plants. Acid rain changes the pH of the land where the plant is growing, thereby affecting the overall growth of...
What is acid rain made of?
Acid rain comprises highly acidic water droplets due to air emissions, specifically the disproportionate levels of sulphur dioxide and nitrogen dio...
What is the primary source of acid rain?
The power plants primarily cause acid rain. It releases most of the sulphur dioxide and nitrogen dioxide while burning fossil fuels. Sulphur dioxid...
Can acid rain damage buildings?
Yes, acid rain harms buildings. It strips away the materials and corrodes the metals of the buildings. Example: Tarnishing of Taj Mahal.
What happens when acid rains?
Acid rain also causes the corrosion of water pipes. Which further results in leaching of heavy metals such as iron, lead and copper into drinking water. It damages the buildings and monuments made up of stones and metals.
What is acid rain made of?
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. Often called acid rain as this concept contains many types of acidic precipitation. The acidic deposition takes place in two ways: wet, and dry.
What is the impact of acid rain on Taj Mahal?
The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as a domestic fuel, adding to this problem. Acid rain has the following reaction with the marble ( calcium carbonate ):
How can we prevent acid rain?
Prevention of Acid Rain 1 The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur. 2 We have so far seen the details of acid rain and its harmful effect on animals, plants and the monuments. 3 Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
How does acid rain affect agriculture?
Acid rain affects agriculture by the way how it alters the composition of the soil. It causes respiratory issues in animals and humans. When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. As it alters the chemical composition of the water, to a form which is actually harmful to ...
How are sulphur and nitrogen mixed?
Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions are given out from industries or by natural causes like how a lightning strike in the atmosphere releases nitrogen ions and sulphur is released from volcanic eruptions.
What are some examples of oxidation reactions?
Example – the burning of fossil fuels, unethical waste emission disposal techniques . Sulphur dioxide and nitrogen dioxide undergo oxidation, and then they react with water resulting in the formation of sulphuric acid and nitric acid respectively. The following reaction will clarify the acid formation reaction:
Causes of Acid Rain
Acid rain is caused by many different things, such as burning fossil fuels and the use of aerosols.
Acid Rain Caused By Pollution
Pollution is one of the main causes of Acid Rain. It’s an often overlooked villain, and can be found all over North America as well as other parts of the world.
Acid Rain Caused By Industries
It is very important for us to know the causes of Acid Rain like Acid rain caused by industries is a problem that has been going on for decades. The effects of acid rain are devastating to both humans and the environment, so more must be done in order to combat this issue.
Acid Rain Caused By The Burning
Causes of acid Rains is an on going debate nut one thing is sure burning is a key factor in all this.
Acid Rain Caused By Carbon Dioxide
One of the primary causes of acid rain is carbon dioxide, a toxic gas that has been responsible for global warming and climate change.
Acid Rain Caused By Volcanoes
Volcanoes are the ultimate producers.
Acid Rain Caused By Nuclear Radiation
Nuclear radiation has been known to have a negative effect on the environment and human health.
The Causes of Acid Rain
The bad thing about acid rain is that unlike other types of rain, it’s harmful to the environment and living creatures.
The Impact of Acid Rain
The industrial revolution of the late 18th and through the 19th centuries brought many beneficial devices to our lives, but in parallel it also caused a great amount of pollution.
Ways to Reduce and Prevent Acid Rain
We are all the aware of the negative effects that come with environmental pollution since it is a prominent issue to deal with nowadays.
Why Is This Bad for Your Health?
So, you might have heard about the phenomenon called acid rain, how it is bad for your health and the environment. But do you know what it is?
The Risks of Acid Rain on Your Home's Exterior
As you can imagine, acid rain causes a lot of damage to buildings. You’ve probably noticed that the stone of your building is sometimes eroded or dissolved.
What Can We Do to Keep Our Environment Safe?
What should we do? I mean, do we even have a choice. The answer is: Yes, yes, we do.
What is the result of acid rain?
Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
Where does acid rain come from?
While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO 2 and NO X in the atmosphere are: Burning of fossil fuels to generate electricity.
What is acid rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
How does acidic water affect the environment?
When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
Is rain acidic or alkaline?
The lower a substance's pH (less than 7), the more acidic it is; the higher a substance's pH (greater than 7), the more alkaline it is. Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO 2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.
What Is Acid Rain?
The acid levels of rain are measured on the pH-scale. (Foto: CC0 / Pixabay / PublicDomainPictures)
How is Acid Deposition Measured?
Different authoritative sources offer disparate information on the exact pH-levels of acidic rain or acid deposition, with estimates ranging anywhere from 3.0 to 5.6. To better understand these numbers, note some of the basics of measuring acidity and alkalinity: The pH-scale ranges from 0 (acidic) to 14 (alkaline/basic).
How Does Acid Rain Form?
Coal-burning power plants are among the primary causes of acid rain. (Foto: CC0 / Pixabay / Benita5)
What are the Effects of Acid Rain?
Acid rain takes a serious toll on the environment. Forests and aquatic areas such as streams, rivers, and lakes are especially at risk, as is wildlife.
Acid Rain: What Has Been Done About It and What Still Needs to Be Done?
The causes of acid rain outlined above were much more pervasive in the past before new pollution-reducing legislation such as the Clean Air Act (1970) was introduced in the United States. Over the years, many other countries also significantly reduced the emission of sulfur from various sources, for example, by retrofitting power plants.
What causes acid rain?
But, it is mainly caused by the combustion of fossil fuels which results in emissions of sulfur dioxide (SO 2) and nitrogen oxides (NO x ). 1.
Why is rain water acidic?
Normal rainwater is slightly acidic with a pH range of 5.3-6.0 because carbon dioxide and water present in the air react together to form carbonic acid, which is a weak acid. When the pH level of rainwater falls below this range, it becomes acid rain.
What gases react with water and oxygen to form acidic compounds?
These gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds such as sulfuric acid, ammonium nitrate, and nitric acid. As a result, these areas experience exceedingly high amounts of acid rain.
What is acid rain?
According to EPA, “ Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic. ”.
What is the name of the gas that makes the air acidic?
It is easily defined as rain, fog, sleet or snow that has been made acidic by pollutants in the air as a result of fossil fuel and industrial combustions that mostly emits Nitrogen Oxides (NOx) and Sulfur Dioxide (SO2).
Why is acid rain bad for humans?
Human health is not directly affected by acid rain because acid rainwater is too dilute to cause serious health problems.
How does acid rain affect biodiversity?
Acid rain runoff from catchment areas into rivers and lakes has also reduced biodiversity as rivers and lakes become more acidic. Species including fish, plant and insect types in some lakes, rivers and brooks have been reduced and some even completely eliminated owing to excess acid rain flowing into the waters. 2.
Why is acid rain so severe?
The phenomenon of acid rain is especially severe in areas that have a high population density compared to rural areas since the number of inhabitants usually positively correlates with the number of cars and therefore leads to more emissions and eventually to more acid rain.
What is acid rain?
Acid rain can be defined as rain or any other kind of precipitation that is unusually acidic, which means that it has higher levels of hydrogen and thus a lower pH-score. It is caused by emissions of nitrogen oxide and sulfur dioxide, which react with water molecules in the atmosphere and produce acid rain. Acid rain has harmful effects on the ...
How does acid rain affect the environment?
Moreover, also humans are adversely affected since acid rain causes corrosion of steel structures (e.g. bridges) and the weathering of statues and stone buildings.
What are the effects of acid rain?
Effects of Acid Rain 1 Effects on aquatic environments 2 Effects on animals and plants 3 Effects on forests 4 Effects on global warming 5 Effects on soil 6 Effects on vegetation cover 7 Effects on buildings 8 Effects on health
Why do trees need pH?
Forests, like many plants, need a certain pH-level to grow in an optimal way. If acidity levels are altered due to acid rain, trees may no longer be able to grow. Moreover, the trees and the corresponding ecosystem are more vulnerable to insect destruction, diseases, and damages caused by extreme weather.
How does acid rain affect water?
Also, the groundwater system is eventually affected by acid rain. This leads to an overall drop in pH-levels in the entire water system.
Why is agriculture important in acid rain?
Agriculture also plays an important role as a factor for acid rain. On the one hand, farmers often use excessive amounts of fertilizers and pesticides in order to maximize their crop yields. However, these substances can contain substances like nitrogen compounds which can eventually result in acid rain.
