
3 causes of corrosion you should probably know all about
- Weather Conditions. The main cause of corrosion on most of the metals is its exposure to weather conditions. ...
- Regions. Your metal objects will corrode faster if you live in coastal regions. If you live in coastal regions your metal objects will corrode faster.
- Neglect. Another common cause of corrosion is neglect. ...
What are the main causes of corrosion?
Causes Of Corrosion • Some of the main and popular causes of corrosion are as follows: • Too much humidity or condensation of water vapour on metal surfaces are the primary causes of corrosion. • Corrosive gases such as chlorine, hydrogen oxides, ammonia, sulfur oxides, amongst others can result in corrosion of parts of electronic equipment, etc. Corrosion can also occur due to hydrogen ...
How do you prevent corrosion?
You can prevent corrosion by selecting the right:
- Metal Type
- Protective Coating
- Environmental Measures
- Sacrificial Coatings
- Corrosion Inhibitors
- Design Modification
How do you remove corrosion from metal?
Use abrasive tools to scrape the rust off.
- Steel wool is easy to use and something you may already have in your home.
- Use an electric sander to remove rust on large pieces. ...
- Any metal tool can be used to scrape metal, but use a fine grain sandpaper afterwards to try to remove any marks the scraping may have made.
What are the differences between corrode and erode?
is that erode is to wear away by abrasion, corrosion or chemical reaction while corrode is to eat away by degrees; to wear away or diminish by gradually separating or destroying small particles of, as by action of a strong acid or a caustic alkali.
What are the 3 main causes of corrosion?
3 causes of corrosion you should probably know all aboutFrom vehicles to metal tools, machinery to gutters of our homes, you will find corrosion everywhere. Corrosion and rust is the result of aging metals. ... Regions. Your metal objects will corrode faster if you live in coastal regions. ... Neglect.
What causes corrosion in Class 10?
The gradual destruction of pure metals by the action of air, moisture or a chemical (such as an acid) on their surface is called corrosion. When an iron object is kept in damp air for a considerable time , then a red-brown substance called rust is formed on its surface. The Corrosion of Iron is called 'Rusting'.
What are the causes and effects of corrosion?
Too much humidity or condensation of water vapour on metal surfaces are the primary causes of corrosion. Corrosive gases such as chlorine, hydrogen oxides, ammonia, sulfur oxides, amongst others can result in corrosion of parts of electronic equipment, etc. Corrosion can also occur due to hydrogen and oxygen exposure.
What is corrosion 11th class?
The corrosion of a metal is defined as a natural process that converts a refined metal into a more chemically stable form such as oxide, hydroxide, or sulphide. It is basically the gradual destruction of metal by chemical and/or electrochemical reaction with the environment.
What is corrosion Class 10 SSC?
Corrosion is the damage caused to the metal by the chemical reaction of air, water and acids with the surface of the metal.
What is corrosion class 10 Brainly?
Hello! Corrosion is the process of rusting of metal or alloy due to the presence of moisture and air in it. It is a irreversible damage or destruction and natural process.
What is corrosion class 10 prevention?
Prevention of Rusting 1) By painting. 2) By applying grease or oil. 3) By galvanisation : The process of depositing a thin layer of zinc metal on iron. 4) By tin plating and chromium plating. 5) By alloying it.
What is corrosion very short answer?
Corrosion is when a refined metal is naturally converted to a more stable form such as its oxide, hydroxide or sulphide state this leads to deterioration of the material.
What is corrosion? Explain with an example.
The eating of metals by their own oxides by the action of air, water, and chemical reaction is called corrosion. The most common example of corrosi...
Do all metals corrode?
All metals do not corrode. The metals that are placed higher in the reactivity series such as iron, zinc etc., corrode very easily whereas metals p...
What are the methods to prevent corrosion?
The methods to prevent corrosion are as follows: 1. Alloying 2. Galvanization 3. Electroplating 4. Painting and greasing.
What causes corrosion?
Some of the causes are as follows: 1. Metal corrodes when it reacts with another substance such as oxygen, hydrogen, an electrical current or even...
What is corrosion and its effects?
The eating of metals by their own oxides by the action of air, water and chemical reaction is called corrosion. The effects of corrosion are that i...
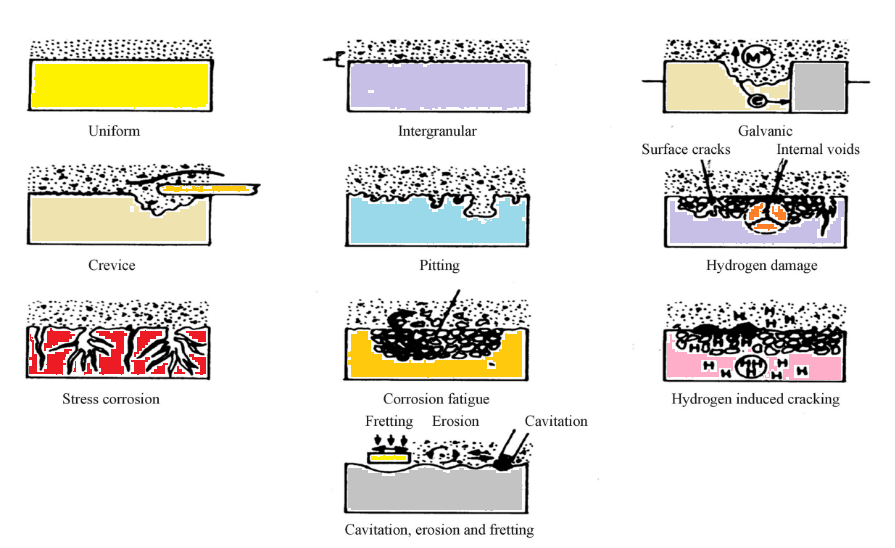
What are the three types of corrosion?
The three types of corrosion are- General corrosion- It is an electrochemical corrosion. It occurs as a result of the rust. When metal, specificall...
Do non-metals undergo corrosion?
No, only active metals undergo the process.
What is the condition necessary for corrosion?
The condition necessary for corrosion to take place is moisture. This can be in the form of moist air, oxygen, and water.
Explain the corrosion of Aluminum metal.
Aluminum being a reactive metal does not corrode like other metals. It forms a protective layer of aluminum oxide on corrosion which prevents the m...
What causes corrosion in metal?
Some of the main and popular causes of corrosion are as follows: 1 Too much humidity or condensation of water vapour on metal surfaces are the primary causes of corrosion. 2 Corrosive gases such as chlorine, hydrogen oxides, ammonia, sulfur oxides, amongst others can result in corrosion of parts of electronic equipment, etc. Corrosion can also occur due to hydrogen and oxygen exposure. 3 Corrosion in steel can occur when it is placed under too much stress and the material develops a crack in it. 4 Metals exposed to electrical currents for a long time can experience electronic corrosion. 5 Exposure to dirt and bacteria can cause corrosion in metals.
Why does corrosion occur in steel?
Corrosion in steel can occur when it is placed under too much stress and the material develops a crack in it. Metals exposed to electrical currents for a long time can experience electronic corrosion. Exposure to dirt and bacteria can cause corrosion in metals.
What gases cause corrosion?
Corrosive gases such as chlorine, hydrogen oxides, ammonia, sulfur oxides, amongst others can result in corrosion of parts of electronic equipment, etc. Corrosion can also occur due to hydrogen and oxygen exposure.
How Corrosion Occurs?
Corrosion is an electrochemical reaction that appears in several forms, such as chemical corrosion and atmospheric corrosion, the latter of which is the most common form. When acidic substances (including water) come in contact with metals, such as iron and/or steel, rust begins to form. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture (e.g., humidity, vapor, immersion). When steel is exposed to water, the iron particles are lost to the water’s acidic electrolytes. The iron particles then become oxidized, which results in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and flow through the steel to another area of the steel known as the cathodic area.
How Can I Prevent Corrosion?
One of the best ways to prevent corrosion is to apply an Anti-Corrosion Protective Coating. A protective coating protects its substrate by preventing contact between the substrate and harsh environments (atmospheric, chemical, etc.). Here at CSL Silicones Inc, we offer two kinds of anti-corrosion protective coatings (one is an environmentally responsible Low VOC option!) that are easily applied using only one coat. The Si-COAT® 579 AC protective coating is cost-effective and offers long-lasting protection to virtually any substrate.
1. Natural and man-made environmental causes
The first thing to examine is the equipment's environment, both natural and man-made. Exposure to certain elements induces the corrosive process, so it is important to limit the time your aircraft spends in or near such hazards. First, let's look at the key man-made factors:
2. Corrosive cells
Aside from the aforementioned environmental factors, a leading cause of corrosion is the material selection itself. Placing dissimilar metal surfaces in contact with one another creates problems. On metal surfaces, atoms tend toward oxidation and lend electrons to oxygen molecules in the surrounding environment.
Conclusion
Understanding the causes of corrosion allows you to optimize operational conditions for your aircraft to best combat corrosive effects. An understanding of the chemistry behind corrosion will help you formulate the ideal treatment and maintenance plan to fit your local conditions and your particular equipment.
What are the factors that affect corrosion?
1. Moisture- Metals tend to corrode at a much faster rate in moist or wet environments. 2. Temperature- An increase in temperature increases the rate of corrosion. 3. Exposure of metals to air conditioning gases such as C O 2, S O 2, S O 3, etc. increases the rate of corrosion. 4.
Why is corrosion a major challenge?
Corrosion is a significant challenge because it causes disasters and massive financial loss. Prevention of corrosion is important because the majority of the materials and structures we use are made of metals. Some of the preventive measures are as follows:
What are the different types of corrosion?
Some of the general types of corrosion are as follows-. 1. Galvanic corrosion: This type of corrosion occurs when two metals with different electrochemical charges are linked via a conductive path. It occurs when metal ions move from the anodic metal to the cathodic metal.
What is the process of eating metals by their own oxides?
Corrosion degrades the useful properties of materials and structures including strength, appearance, and permeability to liquids and gases. In other words, it is a gradual process of eating of metals by their own oxides that results in the decaying or deterioration of metal or metal surface.
What is the term for the rusting of iron?
Rusting. Corrosion of iron metal is called rusting. When iron or alloys of iron reacts with moist air present in the atmosphere, they form a reddish-brown layer of iron oxide, F e 2 O 3 (rust). Rusting of iron causes severe damage to the iron substance.
What type of corrosion occurs when one impure metal is present?
2. Intergranular corrosion: This type of corrosion occurs when one impure metal is present. If a metal contains a combination of alloys that possess different charges, one of the metals can become corroded.
What is the process of converting a refined metal into a stable chemical form?
Corrosion is a natural process that converts a refined metal into a stable chemical form such as oxide, hydroxide, or sulphide. It is the gradual decomposition of materials (usually metals) as a result of chemical and/or electrochemical reactions with their surroundings.
