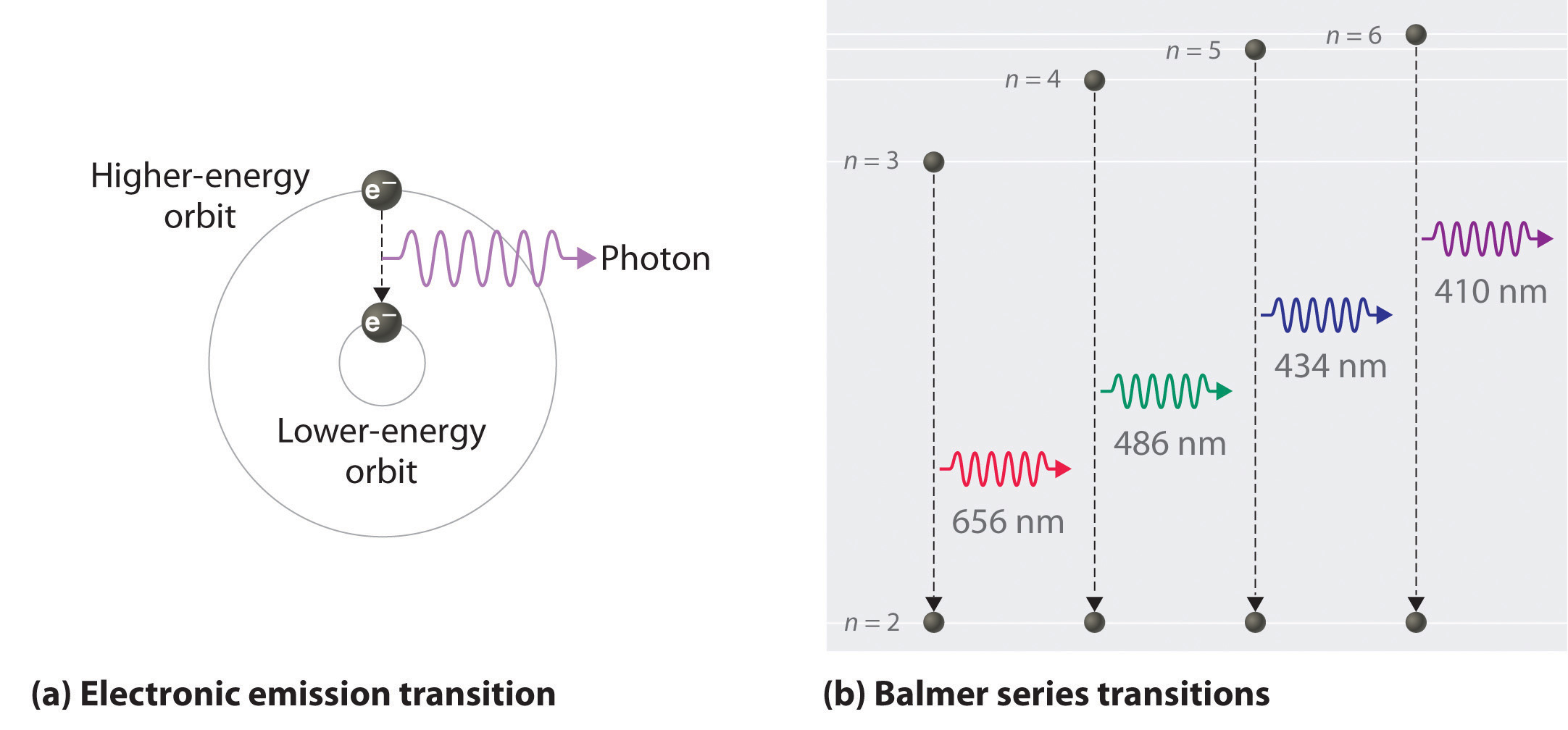
The visible spectrum of light from hydrogen displays four wavelengths, 410 nm, 434 nm, 486 nm, and 656 nm, that correspond to emissions of photons by electrons in excited states transitioning to the quantum level described by the principal quantum number n equals 2.
See more
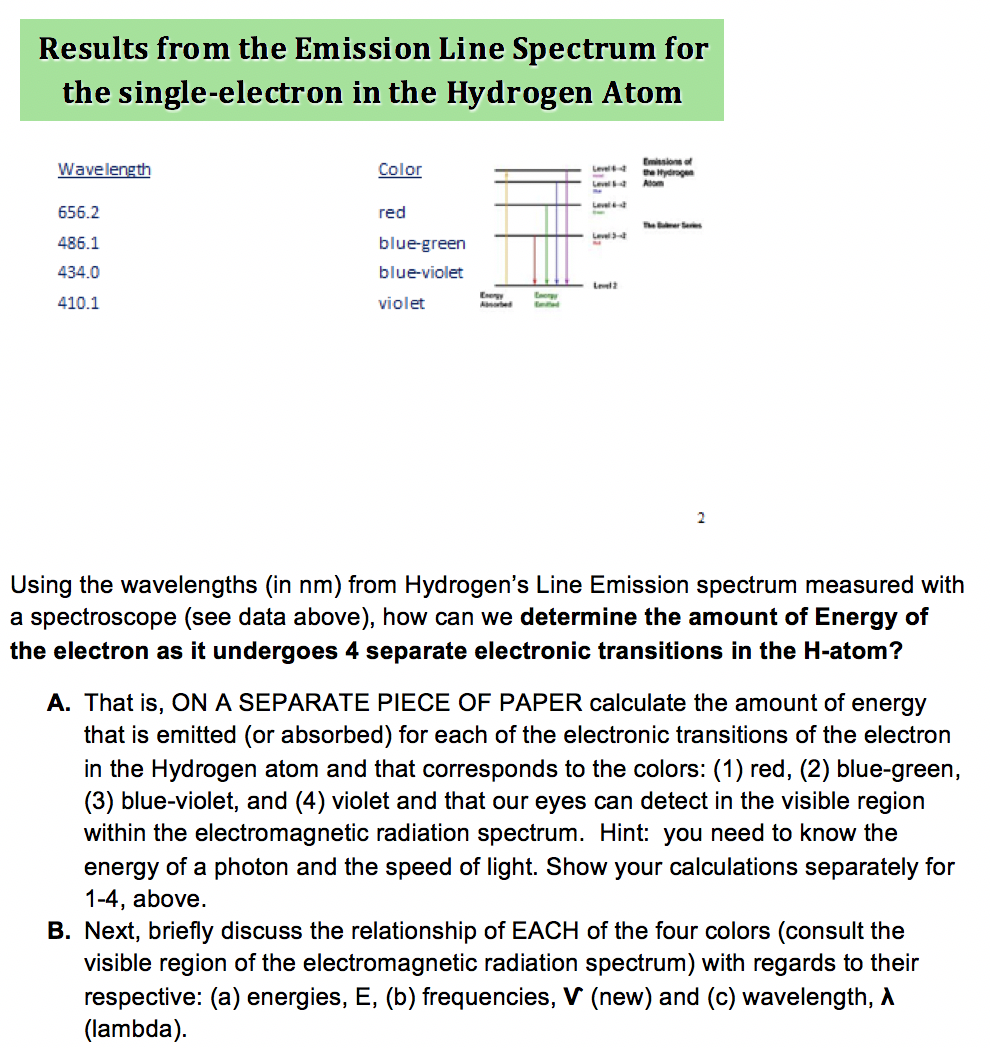
Why are there only 4 lines in the emission spectrum of hydrogen?
Why does the hydrogen spectrum give many lines? Whilst hydrogen has only one electron, there are many shells or energy levels which that electron can transition between. The gaps between those levels are all different, so each has its own frequency resulting in different lines in the spectra.
How many lines are there in the emission spectrum in the visible region for hydrogen?
four characteristic linesThe light emitted by hydrogen atoms is red because, of its four characteristic lines, the most intense line in its spectrum is in the red portion of the visible spectrum, at 656 nm.
Which series of lines of hydrogen spectrum appears in visible region?
the Balmer seriesThis series of the hydrogen emission spectrum is known as the Balmer series. This is the only series of lines in the electromagnetic spectrum that lies in the visible region.
What are the lines of emission spectrum hydrogen represent?
The so-called Lyman series of lines in the emission spectrum of hydrogen corresponds to transitions from various excited states to the n = 1 orbit. Calculate the wavelength of the lowest-energy line in the Lyman series to three significant figures.
What are the 5 series of emission line spectrum?
The main spectral series are the Lyman, Balmer, Paschen, Brackett, Pfund, and Humphreys.
How many lines are formed in the visible region of spectrum?
Three spectral lines will be obtained in the line spectrum.
Are all Balmer lines in visible region?
Four of the Balmer lines are in the technically visible part of the spectrum, with wavelengths between 400nm−700nm.
Why Balmer series is visible region?
The "visible" hydrogen emission spectrum lines in the Balmer series. H-alpha is the red line at the right. Four lines (counting from the right) are formally in the visible range. Lines five and six can be seen with the naked eye, but are considered to be ultraviolet as they have wavelengths less than 400 nm.
Which of the following series of lines is found in visible?
Balmer seriesBalmer series is seen in the visible region.
What do the emission lines represent?
Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. When light passes through gas in the atmosphere some of the light at particular wavelengths is scattered resulting in darker bands.
Why are there multiple lines in the hydrogen line spectrum?
Though a hydrogen atom has only one electron, it contains a large number of shells, so when this single electron jumps from one shell to another, a photon is emitted, and the energy difference of the shells causes different wavelengths to be released... hence, mono-electronic hydrogen has many spectral lines.
What is the brightest hydrogen emission line in the visible spectrum?
H-alphaH-alpha (Hα) is a specific deep-red visible spectral line in the Balmer series with a wavelength of 656.28 nm in air and 656.46 nm in vacuum; it occurs when a hydrogen electron falls from its third to second lowest energy level. H-alpha light is the brightest hydrogen line in the visible spectral range.
What is the wavelength of the line in the spectrum of hydrogen?
Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental value of 486.1 nm for the blue line in the visible spectrum of the hydrogen atom.
Why are there so many lines in hydrogen spectrum Class 11?
A hydrogen atom has only one electron, yet it contains a large number of shells. So, when this single electron jumps from one shell to another, a photon is emitted, and the energy difference of the shells causes different wavelengths to be released. Hence, mono-electronic hydrogen has many spectral lines.
How is the hydrogen emission spectrum formed?
Firstly a hydrogen molecule is broken into hydrogen atoms. The electron in a hydrogen atom absorbs energy and gets excited. The electron jumps from...
What are the first three series of hydrogen emission spectrum?
Lyman, Balmer and Paschen series
What is the formula to find out the wavenumber of a radiation in the emission spectrum of hydrogen?
\(\begin{array}{l}⊽=109677\left ( \frac{1}{n_{1}^{2}} -\frac{1}{n_{2}^{2}}\right )\end{array} \)
Name the series which falls in the visible region of the hydrogen emission spectrum?
Balmer series. It is a hydrogen spectral series found in the hydrogen spectrum.
How does an electron travel around the nucleus?
The electron in a hydrogen atom travels around the nucleus in a circular orbit . The energy of the electron in an orbit is proportional to its distance from the nucleus. The further the electron is from the nucleus, the more energy it has. Only a limited number of orbits with certain energies are allowed.
What is the frequency of red light?
Red light with a wavelength of 700.0 nm has a frequency of 4.283 x 1014s-1. Substituting this frequency into the Planck-Einstein equation gives the following result.
Which equation states that the energy of a photon is proportional to its frequency?
Planck's equation states that the energy of a photon is proportional to its frequency.
What happens when hydrogen atoms are activated?
But a higher level for hydrogen atom is not stable so it will jump down to a lower energy level quickly, thereby emits photon and forms hydrogen spectrum.
How many transistions are there in a second?
Now in one second there are a billion nano seconds, so about a billion transistions are taking per second in one single atom. And so some transistions are more probable and some are less, but all will happen.
Which orbits revolve around the nucleus?
electrons revolve around nucleus in specific orbits (energy levels). these orbits are the least possible energy levels for the atom. like in hydrogen atom electron revolve in first orbit (energy level). i
What is the basic state of hydrogen?
Under normal situation, most of the hydrogen atoms are in the basic state (n=1), although a tiny parts of the entirety will occupy some higher energy level (n>1). When activated, hydrogen atom will absorb enough energy to have a level-up transition, for example, from n=1 to n=3.
Why are there two electrons in a central field?
Because, as others have noted, there are two electrons, which means there are many more energy levels. Further, if we just consider the excitation of one, the remaining one breaks the symmetry of the excited state. The motion in a central field gives energy levels independent of angular momentum (leaving aside some special second order effects) but with another electron below the field can no longer be described as just central. There are also a number of other possibilities, such as spin-spi...
How long does an electron stay in the higher energy level?
And since this electron cannot stay in the higher energy level for more than a few nano seconds , it immediately comes down.
Why are there so many different emission lines?
So because there are several orbital levels, there are many combinations possible, each producing different emission lines. At earthly temperatures, many of these combinations do not occur, but in stars or supernovae or other much hotter objects, they appear.
