Isomers of Pentane It contains three structural isomers including neopentane, isopentane, and n-pentane. The formulas of these three isomers of pentane are as follows
What do the isomers of pentane always have?
There are three isomers of pentene: 1-pentene, cis-2-pentene, and trans-2-pentene. An isomer is when a compound has the same chemical structure but the atoms are arranged differently. Pentene isomers include one with the double bond at carbon 1 and two with the double bond at carbon 2. Click to see full answer.
How many structural isomers does pentane have?
Pentane has three structural isomers. The way the atoms in the pentane isomers are arranged is different. The physical properties of these isomers, like their melting and boiling points, are different. The chemical properties of structural isomers are all the same. Pentane's isomers are: N-pentane. Isopentane. Neopentane.
Which isomer has higher boiling point?
You will notice that: the trans isomer has the higher melting point; the cis isomer has the higher boiling point. Subsequently, question is, does pentane or hexane have a higher boiling point? Pentane is straight chain alkane and neopentane is branched chain alkane with same molar mass.
What are the possible isomers?
Types of structural isomerism
- Chain isomerism. These isomers arise because of the possibility of branching in carbon chains. ...
- Position isomerism. In position isomerism, the basic carbon skeleton remains unchanged, but important groups are moved around on that skeleton.
- Functional group isomerism. ...
What is the feedstock for alkylation?
What is the chemical name for deep catalytic cracking?
What is 1-pentene made of?
What is the chemical formula for pentenes?
Who makes 1-pentene?
See 2 more
About this website
How many isomers are of pentene?
five possibleThere are five possible isomers of Pentene.
How many isomers does C5H10?
C5H10 is the molecular formula' of 13 hydrocarbon isomers (represented by their CAS numbers on the chart). They can be divided into cycloalkanes and alkenes.
How many cyclic isomers are there for pentene?
5 isomers are possible.
What are the isomers of alkenes?
Using butene as an example, there are four isomers for the alkene (1-butene, cis-2-butene, trans-2-butene, and isobutene), whereas there are only two for the corresponding alkane (n-butane and isobutane).
How do you calculate isomers?
Total no. of stereoisomers = [ 2(n-1)-2(n/2-1/2)] + 2(n/2-1/2)]
How do you find isomers?
- The number of optical isomers of a compound is determined by calculating the number of chiral centres in it. - The maximum number of optical isomers is given by the formula 2n−1, where n is the number of chiral centres.
What are the 5 isomers of C5H10?
The six possible alkenes i.e. chain/position and geometrical isomers for C 5 H 10 are:Pent-1-ene.(E)Pent-2-ene.(Z)Pent-2-ene.2-Methylbut-1-ene.3-Methylbut-1-ene.2-Methylbut-2-ene.
Does 2-pentene have isomers?
2-Pentene has two geometric isomers, cis-2-pentene and trans-2-pentene.
How do you find the number of isomers of alkenes?
3:5218:45How to calculate isomers tricks:part 1 - YouTubeYouTubeStart of suggested clipEnd of suggested clipThen total number of isomer. Equal 2 to the power n minus 2 plus 1 you have to simply learn thisMoreThen total number of isomer. Equal 2 to the power n minus 2 plus 1 you have to simply learn this formula for even number of even number of carbon atom.
What are the 4 types of isomers?
Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers.
What are the isomers of alkanes?
IsomerismC1: methane only.C2: ethane only.C3: propane only.C4: 2 isomers: butane and isobutane.C5: 3 isomers: pentane, isopentane, and neopentane.C6: 5 isomers: hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane.More items...
What are the 8 isomers?
Isomers are compounds that contain exactly the same number of atoms, i.e., they have exactly the same empirical formula, but differ from each other by the way in which the atoms are arranged.
How many alkene isomers are possible for C5H10?
The six possible alkenes i.e. chain/position and geometrical isomers for C 5 H 10 are: Pent-1-ene.
How do you find the number of isomers of alkenes?
3:5218:45How to calculate isomers tricks:part 1 - YouTubeYouTubeStart of suggested clipEnd of suggested clipThen total number of isomer. Equal 2 to the power n minus 2 plus 1 you have to simply learn thisMoreThen total number of isomer. Equal 2 to the power n minus 2 plus 1 you have to simply learn this formula for even number of even number of carbon atom.
How many non cyclic isomers C5H10?
Originally Answered: how many isomers does c5h10 have? There are a total of 13 isomers with the molecular formula C5H10.
Does C5H10 have stereoisomers?
1 Answer. Truong-Son N. I got 7 in total, including R/S stereoisomers. Since you just mention cyclic C5H10 , we are not restricted to five-membered rings.
How is pentane formed?
Pentane is a naturally occurring hydrocarbon that is present in crude oil and natural gas. It is often extracted from crude oil. Pentane can be col...
How many isomers are there of pentane?
Pentane has three structural isomers. The way the atoms in the pentane isomers are arranged is different. The physical properties of these isomers,...
What are all the isomers of pentane?
Pentane is a 5-carbon alkane that has a total of three structural isomers, which are: N-pentane, which is a straight 5-carbon chain. Isopentane,...
Pentane - Wikipedia
Pentane is an organic compound with the formula C 5 H 12 —that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). ...
What are the uses of pentene? - Answers
Pentene is rarely isolated as a separate compound. Instead, it is most often blended into gasoline or, in a mixture with other hydrocarbons, or alkylated with isobutane to make gasoline.
Pentene Formula & Structure | What is Pentene? - Study.com
Learn about pentene and its formula. Explore three different isomers of pentene, understand their characteristics, see their structure, and study...
Structure of 1-PENTENE (C5H10) - Mol-Instincts
The 2D chemical structure image of 1-PENTENE is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of 1-PENTENE are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon ...
What is the feedstock for alkylation?
Production of fuels. Propylene, isobutene, and amylenes are feedstock in alkylation units of refineries. Using isobutane, blendstocks are generated with high branching for good combustion characteristics.
What is the chemical name for deep catalytic cracking?
Isoamylene is one of three main byproducts of deep catalytic cracking (DCC), which is very similar to the operation of the fluid catalytic cracking (FCC). The DCC uses vacuum gas oil (VGO) as a feedstock to produce primarily propylene, isobutylene, and isoamylene.
What is 1-pentene made of?
Most often, 1-pentene is made as a byproduct of catalytic or thermal cracking of petroleum, or during production of ethylene and propylene via thermal cracking of hydrocarbon fraction s. The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process.
What is the chemical formula for pentenes?
Pentenes are alkenes with chemical formula C. 5H. 10. Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether the double bond has a cis or trans form.
Who makes 1-pentene?
The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process.
How many hydrogen atoms are in a pentene isomer?
Pentene isomers contain one double bond between the two carbon apart from 5 carbon and 10 hydrogen atoms.
How many carbon atoms are in pentene?
Pentene is a compound with five carbon atoms and a double bond. If we break apart the name of the compound, then the structure makes sense. The 'pent-' part means 'five' and refers to the longest chain of five carbon atoms. The “ene” part refers to a double bond somewhere in the molecule. The chemical formula for pentene is:
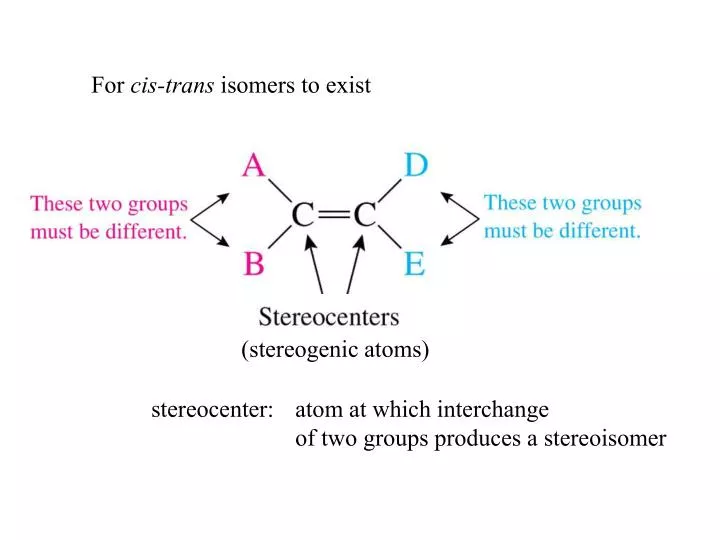
What is the difference between cis and trans?
“Cis” means same or, on this side in Latin, meaning that in cis-2-pentene, the functional groups in the molecule are on the same side, making it a “cis” conformation. “Trans” means across or on the fair side in Latin, meaning that in trans -2- pentene, the functional groups in the molecular are on opposite sides, making it a “trans'' conformation.
What is an isomer?
So, an isomer is when a compound has the same chemical structure but the atoms are arranged differently.
What is Pentane?
Pentane is a colorless, flammable liquid that is lighter than water, and it’s the first liquid member of the alkanes. At 900 parts per million, it has a nice odor, while at 5000 parts per million, it has a moderate odor strength. Isopentane [ (CH 3) 2 CHCH 2 CH 3] and neopentane [C (CH 3) 4] are two more isomers of it.
Structure and Formula of Pentane
Pentane has a straight-chain structure with 5 carbon atoms. Any of the three structural isomers with the chemical formula C 5 H 12 are referred to as pentane. However, in IUPAC nomenclature, the name pentane refers only to the unbranched isomer of the group, n-pentane. Pentane, often known as n-pentane, has the chemical formula C 5 H 12.
Things to Remember
Pentane isomers have boiling temperatures ranging from 9 to 36°C. As with other alkanes, the boiling temperatures of the extensively branched isomers are lower.
Pentene
Pentene is an organic molecule that is entirely composed of hydrogen and carbon atoms. It is a colorless liquid at room temperature with a very unpleasant pungent scent that resembles gasoline. Pentene is an open-chain organic compound with a linear structure.
Pentene Formula
The general pentene formula can be deduced by examining the name "pentene" and recalling the standards the International Union of Pure and Applied Chemistry (IUPAC) has set. The prefix pent- means five, meaning there are five carbon atoms in a pentene molecule. The suffix -ene indicates that the pentene molecule is an alkene.
Isomers of Pentene
Isomers are chemical species that share the same molecular formula but have different structures; the arrangement of atoms differs from one isomer to another. There are two possible placements for the carbon-carbon double bond; it can either be terminal or central.
What is the feedstock for alkylation?
Production of fuels. Propylene, isobutene, and amylenes are feedstock in alkylation units of refineries. Using isobutane, blendstocks are generated with high branching for good combustion characteristics.
What is the chemical name for deep catalytic cracking?
Isoamylene is one of three main byproducts of deep catalytic cracking (DCC), which is very similar to the operation of the fluid catalytic cracking (FCC). The DCC uses vacuum gas oil (VGO) as a feedstock to produce primarily propylene, isobutylene, and isoamylene.
What is 1-pentene made of?
Most often, 1-pentene is made as a byproduct of catalytic or thermal cracking of petroleum, or during production of ethylene and propylene via thermal cracking of hydrocarbon fraction s. The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process.
What is the chemical formula for pentenes?
Pentenes are alkenes with chemical formula C. 5H. 10. Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether the double bond has a cis or trans form.
Who makes 1-pentene?
The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process.

Introduction to Pentane
Isomers of Pentane
- It contains three structural isomers including neopentane, isopentane, and n-pentane. The formulas of these three isomers of pentane are as follows H3C-CH2-CH2-CH2-CH3 (n-pentane) H3C-CH(CH3)-CH2-CH3 (iso-pentane) H3C-C(CH3)3 (neo-Pentane) Before going into details about the properties of each isomer of pentane, let's recall the concept of isomeris...
What Is isomerism?
- Isomerism refers to the phenomenon whereby two chemical compounds have the same chemical formula, with the same kind and number of atoms, but different structural formulas. Due to different structural formulas, their physical properties are also getting different from each other. The molecules that show this phenomenon are called isomers. We can also define isomers as t…
Types of Isomerism
- While studying the phenomenon of isomerism, we encounter two types of isomers; stereoisomers and structural isomers. Stereoisomers are identical to each other in their chemical formulas but different concerning the orientations of their atoms in three-dimensional space. Under this category of stereoisomerism, we also study three more types of isomers named geometrical iso…
Properties of N-Pentane
- n-Pentane is a highly volatile but colorless liquid that is also water-soluble. We get n-Pentane by the fractional distillation of petroleum products. At low concentrations, it forms a flammable and explosive mixture with the surrounding air. On the other hand, at high concentrations, it is used as a narcotic. Speaking of its uses then most of the n-Pentane of the world is utilized in the manufa…
Properties of Iso-Pentane
- Iso-pentane is a watery, colorless, and volatile liquid that possesses a gasoline odor. It's a branched-chain alkane, having five carbon atoms in it. It's also called 2-methyl butane or methyl butane. At room temperature and standard atmospheric pressure, it acts as an extremely flammable liquid and the least dense chemical. That's why the normal boiling point of iso-pentan…
Properties of neo-pentane
- Neo-Pentane is a double-branched chain that is also called 2,2-dimethylpropane. At room temperature and standard atmospheric pressure, it acts as a flammable gas that can be condensed on a highly cold day, or in ice baths, or when compressed at the lowest temperature and highest pressures, into an extremely volatile liquid. The boiling point of neopentane is signifi…
Overview
Pentenes are alkenes with the chemical formula C 5H 10. Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether the double bond has a cis or trans form.
Straight-chain isomers
1-Pentene is an alpha-olefin. Most often, 1-pentene is made as a byproduct of catalytic or thermal cracking of petroleum, or during production of ethylene and propylene via thermal cracking of hydrocarbon fractions.
The only commercial manufacturer of 1-pentene is Sasol Ltd, where it is separated from crude made by the Fischer–Tropsch process.
Branched-chain isomers
The branched isomers are 2-methylbut-1-ene, 3-methylbut-1-ene (isopentene), and 2-methylbut-2-ene (isoamylene).
Isoamylene is one of three main byproducts of deep catalytic cracking (DCC), which is very similar to the operation of the fluid catalytic cracking (FCC). The DCC uses vacuum gas oil (VGO) as a feedstock to produce primarily propylene, isobutylene, and isoamylene. The rise in demand for po…
Production of fuels
Propylene, isobutene, and amylenes are feedstock in alkylation units of refineries. Using isobutane, blendstocks are generated with high branching for good combustion characteristics. Amylenes are valued as precursors to fuels, especially aviation fuels of relatively low volatility, as required by various regulations.