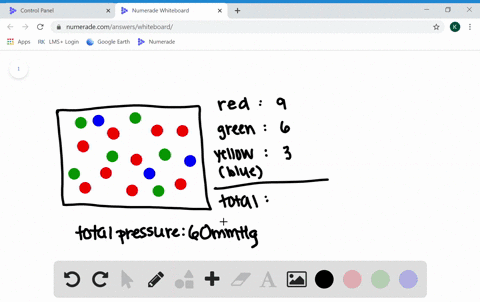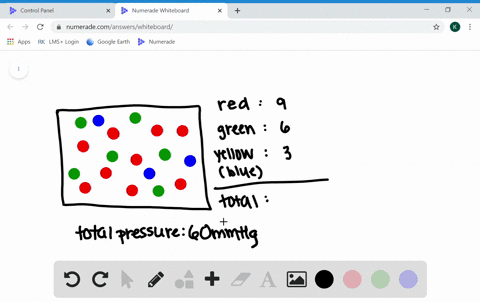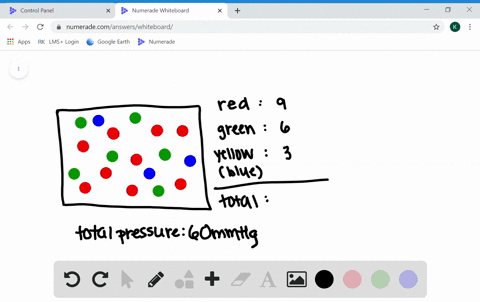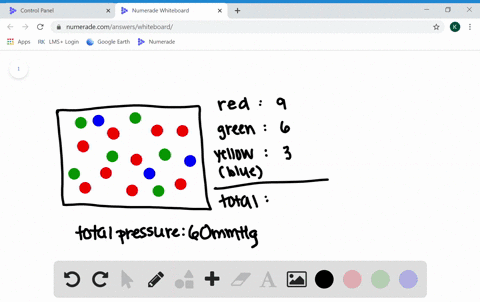
What are the factors affecting the behavior of gases?
When we talk about ideal gases, the following assumptions are taken into consideration:
- The ideal gases are made up of molecules which are in constant motion in random directions.
- The molecules of an ideal gas behave as rigid spheres.
- All the collisions are elastic.
- The temperature of the gas is directly proportional to the average kinetic energy of the molecules.
How does the Charles law explain the behavior of gases?
Charles’s law. If the temperature of a gas is increased, a constant pressure may be maintained only if the volume occupied by the gas increases. This will result in greater average distances traveled by the molecules to reach the container walls, as well as increased wall surface area.
What gases are bad for humans?
Some, but by no means all, toxic gases are detectable by odor, which can serve as a warning. Among the best known toxic gases are carbon monoxide, chlorine, nitrogen dioxide and phosgene .
Why do gases behave ideally?
- Real gases have small attractive and repulsive forces between particles and ideal gases do not.
- Real gas particles have a volume and ideal gas particles do not.
- Real gas particles collide in-elastically (loses energy with collisions) and ideal gas particles collide elastically.
What are the behavior of gases?
The behavior of gases can be modeled with gas laws. Boyle's law relates a gas's pressure and volume at constant temperature and amount. Charles's law relates a gas's volume and temperature at constant pressure and amount. In gas laws, temperatures must always be expressed in kelvins.
What are the 5 properties of gas?
What Are Five Properties of Gases?Low Density. Gases contain scattered molecules that are dispersed across a given volume and are therefore less dense than in their solid or liquid states. ... Indefinite Shape or Volume. Gases have no definite shape or volume. ... Compressibility and Expandability. ... Diffusivity. ... Pressure.
What explains behavior and properties of gases?
The behavior of ideal gases is explained by the kinetic molecular theory of gases. Molecular motion, which leads to collisions between molecules and the container walls, explains pressure, and the large intermolecular distances in gases explain their high compressibility.
What are the 7 properties of gasses?
Properties of GasesWhat are the Properties of Gases? Gasses do not possess any definite volume or shape. ... Compressibility. Particles of gas have huge intermolecular spaces in the midst of them. ... Expansibility. When pressure is exerted on gas, it contracts. ... Diffusibility. ... Low Density. ... Exertion of Pressure.
What is properties of gases?
The Properties of Gases. Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form.
Why do we study the properties and behavior of gases?
Introduction. The study of gases allows us to understand the behavior of matter at its simplest: individual particles, acting independently, almost completely uncomplicated by interactions and interferences between each other.
What is the 4 properties of gases?
Because most gases are difficult to observe directly, they are described through the use of four physical properties or macroscopic characteristics: pressure, volume, number of particles (chemists group them by moles) and temperature.
How is the behavior and properties of gases at the molecular level?
The physical behaviour of gases is explained by the kinetic molecular theory of gases. The number of collisions that gas particles make with the walls of their container and the force at which they collide determine the magnitude of the gas pressure. Temperature is proportional to average kinetic energy.
What are the factors affecting behavior of gas?
Following are the factors that affect the behaviour of gases: Temperature (T) Volume (V) Pressure (P)
What are the properties that help us predict the behavior of gases differentiate one from the other?
Compared to the numbers of molecules involved, there are only a few properties of gases that warrant attention here, namely, pressure, density, temperature, internal energy, viscosity, heat conductivity, and diffusivity.
What is gas?
Gas is a substance which expands freely to fill whatever space it is located. It has no fixed shape and fixed volume. Gas is also called the third...
Explain the compressibility of gases.
Particles of gas have huge intermolecular spaces in the midst of them. By the exertion of pressure, much of this space can be diminished, and the p...
What is the nature of expandability of gases?
When pressure is exerted on gas, it contracts. On the other hand, when pressure is freed, the gas expands.
Explain the nature of diffusibility in gases.
The molecules of the gas are in perpetual motion at a very high velocity. There is a huge amount of intermolecular space amid the molecules. When t...
What is the nature of density in gases?
Since gases have large intermolecular spaces, they have very large volumes when compared to their mass. Therefore, they have very low densities.
Question 1: Describe the Four Properties of a Gas.
Answer: Pressure, volume, temperature, and amount (n) are the properties of a gas.Pressure - The force exerted by the gas molecules on the walls of...
Question 2: What Attributes Decide the Physical Properties of a Matter?
Answer: The following properties decide the physical property of a matter:ColorShapeDensityState or phaseMalleability
Question 3: What is the Viscosity of Gas?
Answer: Viscosity of the gas is the ability of the gas to resist its flow. On rising the pressure, the volume of the gas decreases because of which...
What is gas in science?
A gas is a form of matter that lacks a defined shape or volume. Gases share important properties, plus there are equations you can use to calculate what will happen to the pressure, temperature, or volume of a gas if conditions are changed.
Why are gas laws important?
Important Gas Laws. Because different gases act similarly, it is possible to write a single equation relating volume, pressure, temperature, and quantity of gas. This Ideal Gas Law and the related Boyle's Law, Law of Charles and Gay-Lussac, and Dalton's Law are central to understanding the more complex behavior of real gases.
Why do gases expand?
Expandability - Gases expand to completely fill their containers. Because particles are less ordered than in liquids or solids, the gas form of the same substance occupies much more space . All pure substances display similar behavior in the gas phase. At 0° C and 1 atmosphere of pressure, one mole ...
Why is it easier to compress a gas than to compress a liquid?
Because molecules in a gas are far apart, it is easier to compress a gas than it is to compress a liquid. In general, doubling the pressure of a gas reduces its volume to about half of its previous value. Doubling the mass of gas in a closed container doubles its pressure.
What is the ideal gas law?
Ideal Gas Law: The ideal gas law relates the pressure, volume, quantity, and temperature of an ideal gas. The law applies to real gases at normal temperature and low pressure. PV = nRT. Boyle's Law: At constant temperature, the volume of a gas is inversely proportional to its pressure. PV = k 1.
How many liters of volume is a mole of a gas?
All pure substances display similar behavior in the gas phase. At 0° C and 1 atmosphere of pressure, one mole of every gas occupies about 22.4 liters of volume. Molar volumes of solids and liquids, on the other hand, vary greatly from one substance to another. In a gas at 1 atmosphere, the molecules are approximately 10 diameters apart.
Which law states that at constant pressure, the volume of an ideal gas is directly proportional to temperature?
Charles's law states at constant pressure, the volume of an ideal gas is directly proportional to temperature. Gay-Lussac's law says at constant volume, the pressure of a gas is directly proportional to its temperature. V = k 2 T (Charles's Law), Pi/Ti = Pf/Tf (Gay-Lussac's Law)
What are the properties of gas?
The properties are given below: 1. Compressibility. Particles of gas have huge intermolecular spaces in the midst of them. By the exertion of pressure, much of this space can be diminished and the particles are brought closer. Thus, the volume of gas can be hugely reduced.
What happens when two gases are mixed?
When two gases are mixed, particles of one gas can effortlessly pass through the intermolecular space of the other gas. As an outcome both the gases get completely and consistently mixed.
Why do gases fill all the space?
The characteristic or properties of gases to fill the available volume within a container is the result of the freedom that gas particles have to move everywhere in the accessible space. This autonomy of movement of gaseous molecules is because of the very weak binding forces amidst molecules.
Why are gaseous molecules in continuous motion?
This autonomy of movement of gaseous molecules is because of the very weak binding forces amidst molecules. In other words, their intermolecular forces are very weak. Because of this, the molecules of a gas are in a continuous motion and are related to high velocity and therefore high kinetic energy. The properties are given below: 82,758.
When pressure is exerted on gas, it contracts. When pressure is freed, the gas expands.?
On the other hand, when pressure is freed, the gas expands. When the temperature is augmented, the constituent particles gain more energy, travel faster and move away from each other. Consequently, the intermolecular pull becomes less prominent . The gas’s volume increases.
Which direction do liquids exert pressure?
Solids exert pressure only in the downward direction. Liquids apply pressure downward as well as to the sides. But gases apply pressure in all directions (a good sample is a balloon). This pressure is because of the bombardment of the particles against the walls of the vessel (Figure).
Does decreasing temperature decrease the volume of a gas?
Decreasing the temperature can also decrease the volume of a gas. When the temperature is reduced, there is a lesser amount of energy in the particles; their mobility is diminished and they move less away from each other. As a consequence, the intermolecular pull becomes more prominent and the particles come closer.
What is the property of a gas when it is mixed?
When two gases are mixed, particles of one gas can effortlessly pass through the intermolecular space of the other gas, which is known as diffusion, and this property of a gas is called diffusibility. As an outcome both the gases get consistently and entirely mixed.
Why do gases gain kinetic energy?
If we talk about gases, on rising temperature, the molecules gain super kinetic energy because of which they start colliding with each other and with the walls of the container. However, on the other hand, if we decrease the temperature, the molecules come closer together and the volume of the gas increases with the decrease in its pressure.
How does compression happen?
The compression happens in a way that the molecules set apart in gases come close together and this , in turn, generates a good amount of interatomic force of attraction between these molecules, which is quite similar to the scenario of the molecular spacing and interaction , we observe in liquids. We often use the term "compressibility" in ...
Why do gaseous molecules move in a container?
This determination of movement of gaseous molecules is because of the very weak binding forces among the molecules.
What happens to the volume of a gas when the pressure increases?
Answer: Viscosity of the gas is the ability of the gas to resist its flow. On rising the pressure, the volume of the gas decreases because of which its viscosity increases . However, if we increase the temperature of the gas, the gas expands and its viscosity decreases with the rise in temperature.
What is the motion of gas from one place to another?
The motion of gas from one place to another is related to the velocity of gas molecules. So, the higher is the velocity, greater will be the kinetic energy of gas molecules, which in turn, means a quick flow of gas, as we observe in the PNG gas pipeline system in our kitchens. There are many properties of gases; let’s discuss these one-by-one:
Which direction do solids exert pressure?
As solids exert pressure only in the downward direction liquids apply pressure downward as well as to the sides but gases apply pressure in all directions. A good example is a balloon when we fill the gas inside it, it expands completely.
What is a gas molecule?
Gases consist of loosely bound gas molecules. Each gas molecule may contain one or more atoms bound together. Gases can be classified into monoatomic, diatomic, or triatomic based on the number of atoms in a molecule. In this article, we discuss the chemical and physical properties and the behavior of gas molecules using the kinetic theory of gases.
How to find the moles of gas?
The amount of gas represent by the symbol ‘n’ is the moles of gas present. We can obtain the n value by dividing the mass of the gas by the molar mass of the gas. The higher the value of n, the higher is the number of gas molecules present.
What is the ability of a matter to decrease in volume when under pressure?
Compressibility is the ability of a matter to decrease in volume when under pressure. If we put pressure on a solid or a liquid, then the volume will be changed. The atoms and molecules start to pack-up closer together under pressure and hence the volume decrease.
How does volume affect pressure?
Pressure is affected by the volume of the container. If the volume is decreased, then the gas molecules have less space in which to move around. The frequency of the particles striking the walls of the container will increase. Thus, there will be an increase in pressure. A decrease in gas volume produced increases the gas pressure.
What causes air molecules to move faster?
The kinetic energy causes the air molecules to move faster and they will impact the container walls more frequently and with more force. And eventually, the pressure of the overall container will increase.
What is the behaviour of a gas?
Behaviour of Gas Molecules. The behaviour of gas molecules is dependent on the properties and laws obeyed by the molecules of the gas. The distribution of molecules in a gas is very different from the distribution of molecules in liquids and solids.
What is the classification of gas?
The classification of gases are: Ideal gas. Non-ideal gas or real gas.
What is the ideal gas law?
According to ideal gas law, the product of pressure and volume of one gram molecule of an ideal gas is equal to the product of a number of moles of the gas, universal gas constant and the absolute temperature. Mathematical representation of the law is given in the table: PV = nRT = NkT. Where,
What happens to the volume of gas as the temperature increases?
As the temperature increases, the volume of the gas also increases due to an expansion of gas molecules. As the temperature decreases, the volume of the gas also decreases due to the contraction of gas molecules. As the temperature increases, the pressure of the gas also increases due to an expansion of gas molecules.
What happens to the pressure of gas as the quantity decreases?
As the quantity decreases, the pressure will also decrease and with an increase in the quantity, the pressure also increases. For pressure to decrease, the volume and quantity of the gas should be less. For pressure to increase, the volume and quantity of the gas should be more.
When the volume of a gas is constant, the pressure of a given mass of gas varies directly with the
According to Gay-Lussac’s law, when the volume of the gas is constant, the pressure of a given mass of gas varies directly with the absolute temperature of the gas . Mathematical representation of the law is given in the table:
Is intermolecular interaction negligible?
The intermolecular interactions are also negligible. The collision of molecules with each other and with the walls of the container is always elastic. The average kinetic energy of all the molecules is dependent on the temperature.
What are the properties of gas?
The Properties of Gases. Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers , and (3) they occupy far more space than the liquids or solids from which they form.
What are the characteristics of gas?
Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form.
What happens after a gas cylinder is compressed?
After the gasoline/air mixture is compressed, the spark plug at the top of the cylinder fires and the resulting explosion pushes the piston out of the cylinder in the third stroke. Finally, the piston is pushed back into the cylinder in the fourth stroke, clearing out the exhaust gases. Liquids are much harder to compress than gases.
Why do gases expand to fill their containers?
Because gases expand to fill their containers, it is safe to assume that the volume of a gas is equal to the volume of its container.
How many moles of gas are produced for every 4 moles of liquid that decompose?
Because 29 moles of gas are produced for every four moles of liquid that decompose, and each mole of gas occupies a volume roughly 800 times larger than a mole of liquid, this reaction produces a shock wave that destroys anything in its vicinity. The same phenomenon occurs on a much smaller scale when we pop popcorn.
Which is harder, liquid or gas?
Liquids are much harder to compress than gases. They are so hard to compress that the hydraulic brake systems used in most cars operate on the principle that there is essentially no change in the volume of the brake fluid when pressure is applied to this liquid. Most solids are even harder to compress.
Why is the pressure of the water pushing down on one arm of the U tube equal to the pressure of the alcohol pushing
Because the pressure of the water pushing down on one arm of the U-tube is equal to the pressure of the alcohol pushing down on the other arm of the tube, the system is in balance. This demonstration provides the basis for understanding how a mercury barometer can be used to measure the pressure of the atmosphere.
