Radiation - Interaction With Matter
- This occurs when accelerated particles encounter material that have a high Z number (density). ...
- Accelerated particle is a beta
- Example of a high Z number material would be lead
How does radiation affect matter?
The most destructive effect radiation has on living matter is ionizing radiation on DNA. Damage to DNA can cause cellular death, mutagenesis (the process by which genetic information is modified by radiation or chemicals), and genetic transformation.
How do radio waves interact with matter?
the matter creates its electric or magnetic field and so effects the other matter's magnetic/electric field. now we know that a radio wave is mixture of oscilating electric and magnetic fields and for ex if we put an FM radio near a magnet or electric wire the signals have to weak or diverge... and if we define "light" as an electromagnetic wave, light beam has to deviate while passing near a magnet or electric wire or another electromagnetic wave. i know there sth wrong about my opinion if ...
How does X - ray interact with matter?
X-rays primarily interact with matter through interaction of their oscillating electric field with the atomic electrons in the material. Having no electrical charge, the x-rays are more penetrating than other types of ionizing radiation (such as alpha or beta particles) and are therefore useful for imaging the human body.
How does electromagnetic radiation interact with matter?
Interaction of electromagnetic radiation with matter. Matter can reflect, transmit, or absorb electromagnetic (radiant) energy (Figure 1 above). If electromagnetic radiation is absorbed by matter, then there is a transfer of energy from the radiant energy to the medium that is doing the absorbing. Click to see full answer.
What are the 5 interactions with matter?
Five main interactions can cause attenuation of photons: (1) coherent scattering, (2) photoelectric effect, (3) Compton scattering, (4) pair producion, and (5) photodisintegration.
What are the interactions with matter?
A beam of x-rays may be: Transmitted: pass through unaffected or with a lower energy. Absorbed: transfer all energy to matter and not pass through the patient to the film....Σ Summary.Compton effectPhotoelectric effectInteractions with free / outer shell electronsInteractions with inner shell electrons4 more rows•Oct 10, 2021
Which type of radiation strongly interacts with matter?
Alpha RadiationAlpha Radiation Due to their charge and mass, alpha particles interact strongly with matter, and only travel a few centimeters in air.
What are the three ways electromagnetic radiation interacts with matter?
Photons can travel through a vacuum forever (or at least a very, very long time) until they encounter matter. When photons run into atoms and molecules, there are three fundamental ways in which they interact with the matter: absorption, reflection, and transmission.
How does radiation absorb matter?
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy).
What is it called when radiation interacts with matter and changes direction?
when x-ray photons interact with matter and change direction, the process is called. scatter.
What are the 3 types of radiation?
The three most common types of radiation are alpha particles, beta particles, and gamma rays.
Can electromagnetic radiation interact with matter?
Electromagnetic radiation (EMR) is energy that propagates through vacuum (free space) or through material media in the form of an advancing interaction between electric and magnetic fields. It can make itself manifest by its interaction with matter.
How does beta radiation interact with matter?
When the beta particle moves faster than the speed of light (phase velocity) in the material, it generates a shock wave of electromagnetic radiation known as the Cherenkov radiation. Positrons interact similarly with the matter when they are energetic.
When radiation encounters matter what 3 things happen to it?
These are: absorption (A); transmission (T); and reflection (R). The total incident energy will interact with the surface in one or more of these three ways. The proportions of each will depend on the wavelength of the energy and the material and condition of the feature.
Which type of electromagnetic radiation interacts with matter like a particle?
Photons are a type of boson that carries electromagnetic energy. They have no charge and are therefore an indirectly ionizing particle. At lower energies, they predominantly interact with matter through coherent scatter and the photoelectric effect.
How does ultraviolet interact with matter?
Ultra violet light is just like visible - it causes electronic excitation. However, it is so energetic that a simple jump to a higher level within the atom or molecule starts to go beyond that. The UV photon can provide so much energy that the electron is promoted all the way out of the molecule.
What is interaction between matter and energy?
In natural systems, both energy and matter are conserved within a system. This means that energy and matter can change forms but cannot be created or destroyed. Energy and matter are often cycled within a system, and different forms of matter and energy are able to interact.
How do particles of matter interact with one another?
The particles don't interact with one another but just hit and bounce off of each other when they collide. Develop a model to describe that matter is made of particles too small to be seen. Students are introduced to the idea that matter is made up of tiny particles called atoms and molecules.
How do photons interact with matter?
In this chapter we are mostly concerned with the interactions of electrons and photons with matter, as these are the most commonly used particles in radiotherapy. The dominating feature of any particle is its charge. Electrons carrying a charge of −1.6×10−19 C readily interact via the Coulomb force with other charged particles in the matter they traverse, predominantly with atomic electrons and, to a lesser extent, with protons in atomic nuclei. Photons, on the other hand, carrying no charge, interact relatively rarely with matter. The use of clinical proton beams for radiotherapy is increasing as new facilities are constructed world-wide. As charged particles, proton beams passing through matter behave in a similar way to electrons, that is, they readily undergo interactions with atomic electrons. The difference between proton and electron interactions lies in the proton having a mass roughly two thousand times greater than the electron (1.67×10−27 kg and 9.11×10−31 kg for the proton and electron mass, respectively). The characteristics of proton energy loss in matter makes them highly suitable for radiotherapy, offering distinct advantages over photons and electrons, as will be discussed below. Neutron beams are less often selected as the beam of choice for radiotherapy at the present time, however, they also offer advantages over photon beams for some tumours due to their biological effect on tissue. Being uncharged, neutrons interact in a similar manner to photons and, in fact, produce very similar depth-dose characteristics.
How does ionizing radiation work?
Ionizing radiation, by definition, has sufficient energy to ionize matter. That is, it has sufficient energy to overcome the binding energy of atomic electrons. Radiation of energy below the binding energy of a particular electron shell may still interact with an electron by raising it to a higher, vacant shell (see Chapter 1). As a result of this interaction, the atom has gained energy and is left in an excited state ( Figure 2.1A ). It will eventually lose this excess energy to return to its lowest energy state, or ground state. An electron occupying an outer shell relative to the vacancy may achieve a lower energy state by filling the vacancy ( Figure 2.1C ). The excess energy is released as a characteristic photon (of energy equal to the difference in shell binding energies). If this electron is also in an inner shell, it too will leave behind a vacancy which an outer electron can again occupy ( Figure 2.1C) losing energy in the form of a characteristic photon. This process results in a cascade of electrons moving between shells and a corresponding set of characteristic photons which eventually returns the atom to its ground state.
How do electrons convert kinetic energy into photons?
The conversion of electron kinetic energy into photons as a beam of electrons striking a target is decelerated in the nuclear Coulomb field (bremsstrahlung) is the primary method for obtaining clinical photon beams. As suggested earlier, however, for the normal range of energies considered for diagnostic imaging and radiotherapy (20 keV to 25 MeV), electrons are far more likely to interact through collisions with atomic electrons. The efficiency of this process is therefore generally low. The likelihood of bremsstrahlung depends on the atomic number of the material traversed, Z (the total charge of the nucleus) and the energy of the incident electron, E, according to:
What are the most commonly used particles in radiotherapy?
In this chapter we are mostly concerned with the interactions of electrons and photons with matter, as these are the most commonly used particles in radiotherapy. The dominating feature of any particle is its charge. Electrons carrying a charge of −1.6×10 −19 C readily interact via the Coulomb force with other charged particles in the matter they traverse, predominantly with atomic electrons and, to a lesser extent, with protons in atomic nuclei. Photons, on the other hand, carrying no charge, interact relatively rarely with matter. The use of clinical proton beams for radiotherapy is increasing as new facilities are constructed world-wide. As charged particles, proton beams passing through matter behave in a similar way to electrons, that is, they readily undergo interactions with atomic electrons. The difference between proton and electron interactions lies in the proton having a mass roughly two thousand times greater than the electron (1.67×10 −27 kg and 9.11×10 −31 kg for the proton and electron mass, respectively). The characteristics of proton energy loss in matter makes them highly suitable for radiotherapy, offering distinct advantages over photons and electrons, as will be discussed below. Neutron beams are less often selected as the beam of choice for radiotherapy at the present time, however, they also offer advantages over photon beams for some tumours due to their biological effect on tissue. Being uncharged, neutrons interact in a similar manner to photons and, in fact, produce very similar depth-dose characteristics.
What happens when electrons travel through matter?
The last section was concerned with the dominant interaction that a beam of electrons undergoes when traveling through matter, that of collisions with atomic electrons (in the energy range of interest to radiotherapy, at least). These interactions lead to excitation and ionization of the medium traversed, as represented schematically in Figure 2.2. More rarely, electrons from an incident clinical beam will pass near to and interact with the atomic nucleus, again as a result of the Coulomb force of attraction between negatively charged electron and positively charged nucleus. The path (and momentum) of the incident electron is changed under the influence of the nucleus, resulting in a loss of electron energy. This loss of energy appears as a radiated photon, or x-ray photon (radiative energy loss). The term, bremsstrahlung (‘braking radiation’), is a helpful descriptive name given to this process, shown schematically in Figure 2.4. The probability of this interaction occurring is inversely proportional to the square of the incident particle’s mass. As a result, bremsstrahlung is only significant for electrons. This important process by which x-ray photons can be produced is described further below.
What is collisional and radiative energy loss?
The last section was concerned with the dominant interaction that a beam of electrons undergoes when traveling through matter, that of collisions with atomic electrons (in the energy range of interest to radiotherapy, at least).
What happens when an atom loses an electron?
Where the incoming particle transfers more than the binding energy of an atomic electron to the atom, the electron in question is ejected from the atom, with kinetic energy equal to the total energy transferred, minus the binding energy. As a result of losing an electron, the atom has been ionized (Figure 2.1B).
What are the three phenomena that occur when radiation interacts with matter?
Absorption, transmission and reflection are typical phenomena when radiation interacts with matter.
What happens when radiation hits an object?
When radiation hits an object, different phenomena show how the incident radiation can interact with matter. This includes the following interactions: In order to demonstrate the interaction of radiation with matter, a laser beam is directed onto a white and a black surface, onto a glass pane and onto a mirror.
What is the absorption of energy?
Absorption refers to the taking up of radiant energy by an irradiated object! Figure: Absorption of light on a black and white surface. How strongly an object absorbs the incident radiant energy depends to a large extent on the color of the irradiated surface.
Why does a laser beam change direction?
the light beam is refracted. Such refraction is caused by the fact that the propagation speed of the light changes when it enters the glass pane. In glass, the light propagates at around 30% less speed than in air.
Why is the object still blurred on the metal plate?
However, the fact that the object can still be seen in a blurred form on the metal plate is due to the fact that in reality there is usually no completely diffuse reflection. In most cases it is a mixed form, as the scattered light rays often still have a certain preferred direction.
What happens to the white surface of an object when it absorbs light?
The white surface, on the other hand, absorbs less light and therefore reflects more. The light spot therefore appears larger and more intense. As a result of the absorbed energy, the temperature of the irradiated object increases, since the absorbed energy results in increased particle motion.
Why is the light spot of a laser beam less intense on the black surface compared to the white surface?
Surfaces in dark colors absorb visible light more strongly than bright surfaces . This is also the reason why the light spot of the laser beam is less intense on the black surface compared to the white surface. The light is therefore absorbed more strongly by the black surface and is therefore no longer reflected.
What is radiation physics?
Radiation Physics is the traditional name of the physics field which studies the interaction between ionising radiation and matter, and is particularly interested in the results of these interactions, and the transfer of energy in particular from radiation to the environment [1]. Radiation can be defined as the emission of energy in the form of electromagnetic waves or subatomic particles through a vacuum or in a material medium, which implies an exchange of energy and matter. In other words, radiation is considered energy in motion.
How do neutrons interact with nuclei?
Neutrons can also interact with nuclei through a wide range of nuclear reactions. The processes with the highest probability are reactions of the type (n, p), (n, α), (n, d), etc., in which the incident neutr'on starts from the target nucleus one or more nucleons. In addition, (n, γ) reactions are possible, called radiative capture. The cross sections of these processes are generally proportional to v − 1, which favours such mechanisms in the case of neutrons with low velocity. Superimposed on this smooth behavior is a variable number of resonances, narrow energy ranges in which the cross section passes through a pronounced maximum. These peaks are related to the great stability of the composite nucleus that is formed when the neutr'on is absorbed. As examples of interest in medical physics we can mention the reactions 14 N (n, p) 14C and 1H (n, γ) 2H.
What happens when an electron travels a certain path?
As a consequence of this fact, if an electron travels a certain path s within the material medium in which it moves, it will experience numerous angular deflections, although most of them will be at a small angle. If we ignore the energy losses, it is said that we are in conditions of multiple elastic dispersion. It is therefore convenient to resort to a global description of the change of direction after traveling the distance s. The objective is therefore to determine, given a value of s, the distribution p (Θ; s) of accumulated polar angles of dispersion Θ.
How does ionization occur?
The ionization of internal atomic layers can occur by photon interactions (photoelectric or Compton Effect) or by the impact of charged particles. After ionization, atomic relaxation takes place, that is, the excess energy of the residual excited ion is emitted istropaly in the form of fluorescent radiation. It comprises the characteristic x-rays and the Auger electrons. In Fig. 10 we have represented the mass range ρr0 of electrons, protons and α particles in Al, Cu and Pb.
What is the approximation of Born's approximation with plane waves?
Born's approximation with plane waves predicts that the effective sections (differential and total) of ionization are equal for charge particles Z1e and −Z1e. This oversimplification can be avoided by using the Born approximation with distorted waves. In this formalism, part of the disturbance H′, Eq. (21), is included in the Hamiltonian H0, with which the projectile's zero-order wave function becomes a distorted wave. The new “disturbance”, which is less than H′, is treated first order. Ref. [19] contains a parameterization, based on the Born approximations with plane and distorted waves (relativistic), of the ionization cross-sections of the K, L and M layers of all atoms by impact of electrons and positrons. In Fig. 7 these cross sections for the innermost layers of the Al atom have been represented.
Why does the probability of Compton scattering per interaction with an atom increase linearly with atomic number Z?
The probability of Compton scattering per interaction with an atom increases linearly with atomic number Z because it depends on the number of electrons available for scattering in the target atom. The Klein-Nishina formula describes the angular distribution of photons scattered from a single free electron:
How do gamma rays depend on thickness?
Based on the definition of interaction cross-section, the dependence of gamma rays intensity on the thickness of absorber material can be derived. If monoenergetic gamma rays are collimated into a narrow beam and if the detector behind the material only detects the gamma rays that passed through that material without any kind of interaction with this material, then the dependence should be simple exponential attenuation of gamma rays. Each of these interactions removes the photon from the beam either by absorption or by scattering away from the detector direction. Therefore the interactions can be characterized by a fixed probability of occurrence per unit path length in the absorber. The sum of these probabilities is called the linear attenuation coefficient:
What is the photoelectric effect?
Definition of Photoelectric effect. In the photoelectric effect, a photon undergoes an interaction with an electron which is bound in an atom. In this interaction the incident photon completely disappears and an energetic photoelectron is ejected by the atom from one of its bound shells.
How to calculate attenuation of gamma radiation?
The following equation can then describe the attenuation of gamma radiation. I=I0.e-μx, where I is intensity after attenuation, Io is incident intensity, μ is the linear attenuation coefficient (cm-1), and the physical thickness of the absorber (cm).
What is gamma ray?
Gamma rays are emitted by unstable nuclei in their transition from a high-energy state to a lower state known as gamma deca y. In most practical laboratory sources, the excited nuclear states are created in the decay of a parent radionuclide. Therefore a gamma decay typically accompanies other forms of decay, such as alpha or beta decay. The process of isomeric transition is similar to any gamma emission but differs in that it involves the nuclei’s intermediate metastable excited state (s).
How are X-rays created?
Following a photoelectric interaction, an ionized absorber atom is created with a vacancy in one of its bound shells. An electron from a shell will quickly fill this vacancy with lower binding energy (other shells) or capture a free electron from the material. The rearrangement of electrons from other shells creates another vacancy, which, in turn, is filled by an electron from an even lower binding energy shell. Therefore a cascade of more characteristic X-rays can also be generated. The probability of characteristic x-ray emission decreases as the atomic number of the absorber decreases. Sometimes, the emission of an Auger electron occurs.
What is the effect of Compton scattering?
This deflection decreases the photon’s frequency’s energy ( decrease in photon’s frequency) and is called the Compton effect. The photon transfers a portion of its energy to the recoil electron. The energy transferred to the recoil electron can vary from zero to a large fraction of the incident gamma-ray energy because all scattering angles are possible. A. H.Compton observed the Compton scattering in 1923 at Washington University in St. Louis. Compton earned the Nobel Prize in Physics in 1927 for this new understanding of the particle nature of photons.
What is the interaction between radiation and matter?
You may click on any of the types of radiation for more detail about its particular type of interaction with matter. The different parts of the electromagnetic spectrumhave very different effects upon interaction with matter. Starting with low frequency radio waves, the human body is quite transparent.
How does ultraviolet radiation affect skin?
The near ultravioletis absorbed very strongly in the surface layer of the skin by electron transitions. As you go to higher energies, the ionization energies for many molecules are reached and the more dangerous photoionization processes take place. Sunburn is primarily an effect of uv, and ionization produces the risk of skin cancer.
What is the quantum energy of a microwave?
The quantum energyof microwavephotons is in the range 0.00001 to 0.001 eV which is in the range of energies separating the quantum states of molecular rotationand torsion. The interaction of microwaves with matter other than metallic conductors will be to rotate molecules and produce heat as result of that molecular motion. Conductors will strongly absorb microwaves and any lower frequencies because they will cause electric currents which will heat the material. Most matter, including the human body, is largely transparent to microwaves. High intensity microwaves, as in a microwave oven where they pass back and forth through the food millions of times, will heat the material by producing molecular rotations and torsions. Since the quantum energies are a million times lower than those of x-rays, they cannot produce ionization and the characteristic types of radiation damage associated with ionizing radiation.
What is the quantum energy of infrared light?
The quantum energyof infraredphotons is in the range 0.001 to 1.7 eV which is in the range of energies separating the quantum states of molecular vibrations. Infrared is absorbed more strongly than microwaves, but less strongly than visible light. The result of infrared absorption is heating of the tissue since it increases molecular vibrational activity. Infrared radiation does penetrate the skin further than visible light and can thus be used for photographic imaging of subcutaneous blood vessels.
Is the human body transparent to microwaves?
Most matter, including the human body, is largely transparent to microwaves. High intensity microwaves, as in a microwave oven where they pass back and forth through the food millions of times, will heat the material by producing molecular rotations and torsions.
Does sunlight cause ionization?
While exposure to visible light causes heating, it does not cause ionization with its risks. You may be heated by the sun through a car windshield, but you will not be sunburned - that is an effect of the higher frequency uv part of sunlight which is blocked by the glass of the windshield.
What is the photoelectric effect?
In the photoelectric effect, a photon undergoes an interaction with an electron which is bound in an atom. In this interaction the incident photon completely disappears and an energetic photoelectron is ejected by the atom from one of its bound shells. The kinetic energy of the ejected photoelectron (E e) is equal to the incident photon energy (hν) minus the binding energy of the photoelectron in its original shell (E b ).
When do photoelectrons emit?
Therefore photoelectrons are only emitted by the photoelectric effect if photon reaches or exceeds a threshold energy – the binding energy of the electron – the work function of the material. For very high X-rays with energies of more than hundreds keV, the photoelectron carries off the majority of the incident photon energy – hν.
What is the Compton scattering formula?
Compton scattering formula is the mathematical relationship between the shift in wavelength and the scattering angle of the X-rays. In the case of Compton scattering the photon of frequency f collides with an electron at rest. Upon collision, the photon bounces off electron, giving up some of its initial energy (given by Planck’s formula E=hf), While the electron gains momentum (mass x velocity), the photon cannot lower its velocity. As a result of momentum conservation law, the photon must lower its momentum given by:
What is the wavelength of X-rays?
Most X-rays have a wavelength ranging from 0.01 to 10 nanometers (3×10 16 Hz to 3×10 19 Hz), corresponding to energies in the range 100 eV to 100 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays. The distinction between X-rays and gamma rays is not so simple and has changed in recent decades. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
How far can X-rays travel?
X-rays can travel thousands of feet in air and can easily pass through the human body.
What is Rayleigh scattering?
Rayleigh scattering, also known as Thomson scattering is the low-energy limit of Compton scattering. The particle kinetic energy and photon frequency do not change as a result of the scattering. Rayleigh scattering occurs as a result of an interaction between an incoming photon and an electron, the binding energy of which is significantly greater than that of the incoming photon. The incident radiation is assumed to set the electron into forced resonant oscillation such that the electron re-emits radiation of the same frequency but in all directions. In this case, the electric field of the incident wave (photon) accelerates the charged particle, causing it, in turn, to emit radiation at the same frequency as the incident wave, and thus the wave is scattered. Rayleigh scattering is significant up to ≈ 20keV and like Thomson scattering, is elastic. The total scattering cross section becomes a combination of the Rayleigh and Compton bound scattering cross sections. Thomson scattering is an important phenomenon in plasma physics and was first explained by the physicist J. J. Thomson. This interaction has great significance in the area of X-ray crystallography.
How do charged particles interact with radiation?
For example, charged particles with high energies can directly ionize atoms. On the other hand, electrically neutral particles interact indirectly and transfer some or all of their energies to the matter. This is the key feature of the categorization of radiation sources. They are usually categorized into two general types as follows:
How does beta radiation affect matter?
The nature of the interaction of beta radiation with matter is different from alpha radiation, even though beta particles are also charged particles. Beta particles have a much lower mass compared with alpha particles, and they reach mostly relativistic energies. Its mass is equal to the mass of the orbital electrons with which they are interacting. A much larger fraction of its kinetic energy can be lost in a single interaction than the alpha particle. Since the beta particles mostly reach relativistic energies, the non-relativistic Bethe formula cannot be used. For high-energy electrons, a similar expression has also been derived by Bethe to describe the specific energy loss due to excitation and ionization (the “collisional losses”).
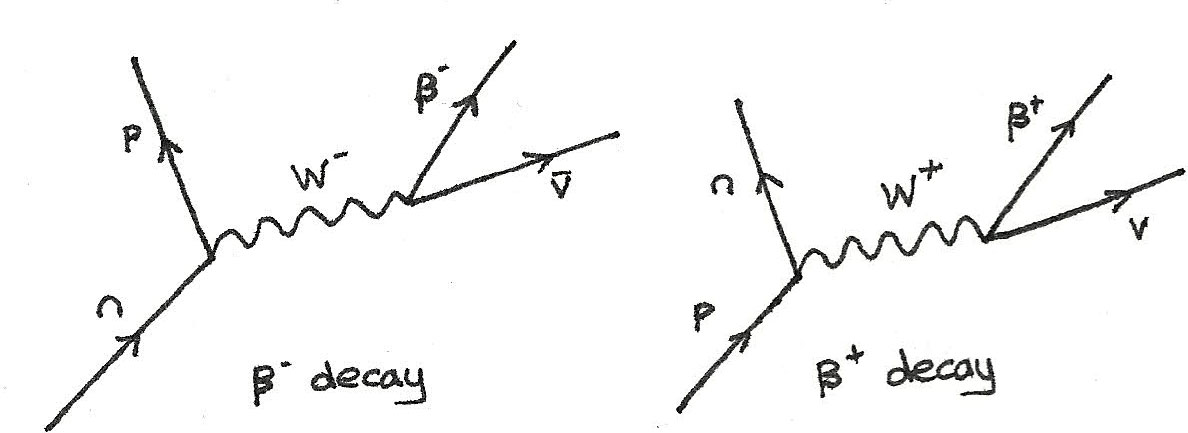
What is the process of producing beta particles?
The production of beta particles is termed beta decay. There are two forms of beta decay , the electron deca y (β− decay) and the positron decay (β+ decay ). In a nuclear reactor occurs especially the β− decay, because the common feature of the fission products is an excess of neutrons ( see Nuclear Stability ).
What happens to fission fragments after nucleus fission?
Fission fragments after nucleus fission. Fission fragments interact strongly with the surrounding atoms or molecules traveling at high speed, causing them to ionize.
What is the mass of a fission fragment?
Fission fragment yield for different nuclei. The most probable fragment masses are around mass 95 (Krypton) and 137 (Barium).
How do beta particles interact?
Moreover, beta particles can interact via electron-nuclear interaction (elastic scattering off nuclei), which can significantly change the direction of a beta particle. Therefore their path is not so straightforward. The beta particles follow a very zig-zag path through absorbing material. This resulting path of the particle is longer than the linear penetration (range) into the material.
What are the particles that are electrically neutral?
Neutrinos . Neutrinos are electrically neutral, weakly interacting elementary particles, which have very low cross sections for any interaction with matter and, therefore, low probabilities for colliding in the matter.

Introduction
Absorption
- While the laser spot on the white surface appears bright, the light spot on the black surface is less intense. Some of the radiant energy is obviously absorbed by the dark surface and is not reflected back afterwards. This phenomenon is therefore called absorption. Absorption refers to the taking up of radiant energy by an irradiated object! How strongly an object absorbs the incident radiant …
Transmission
- A further interaction of radiation with matter occurs when a laser beam hits a pane of glass. While hardly any light spot can be seen on the glass pane, it is clearly visible on a wall behind it. Obviously, hardly any radiant energy is absorbed when passing through the glass, but is almost completely transmitted through it. This phenomenon is therefore called transmission. Transmis…
Reflection
- If a laser beam is directed onto a mirror, there is no spot of light there, but on the opposite wall. The light beam is deflected (almost) without loss of energy. This phenomenon is called reflection. Reflection refers to the throwing back of radiation by a “mirroring” object! In the case of reflection, a distinction can also be made between specular reflection and diffuse reflection.
Interactions in Reality
- Reality shows that absorption, transmission and reflection (both specular and diffuse) are generally not separate but always occur in combination! This can be seen, for example, very clearly with a tinted glass pane. The darkening effect is due to the strong absorption of the light energy by the glass pane. However, the fact that objects can still be seen through the pane show…
Dependence of The Interactions on The Wavelength
- The interactions explained above, such as absorption, transmission and reflection, were illustrated using visible light. However, visible light in a wavelength range from approx. 380 nm to 780 nm actually forms only a small part of the entire electromagnetic spectrum. In principle, the interactions described above apply to all types of electromagnetic radiation. The figure below sh…