Challenges In Gene Therapy
- Gene delivery and activation. For some disorders, gene therapy will work only if we can deliver a normal gene to a large number of cells—say several million—in a tissue.
- Immune response. Our immune systems are very good at fighting off intruders such as bacteria and viruses. ...
- Disrupting important genes in target cells. ...
- Commercial viability. ...
- Gene therapy is not a new field; it has been evolving for decades. Despite the best efforts of researchers around the world, however, gene therapy has seen only limited success. ...
- Gene delivery and activation. ...
- Immune response. ...
- Disrupting important genes in target cells. ...
- Commercial viability.
What obstacles must gene therapy overcome?
What are some of the major challenges that must be overcome to develop safer and more effective viral vectors for gene therapy? -Wrong insertion of DNA during integration. -An immune response to the introduction of viruses.
What are the disadvantages of gene therapy?
What Are the Cons of Gene Therapy?
- It is a costly treatment option. Even if gene therapy becomes an accepted form of treatment for the diseases that affect humanity, the cost of administering them could create ...
- Nature is adaptable. As we have seen with the growing resistance to antibiotics, nature can readily adapt to changes that occur. ...
- It may unlock unethical forms of science. ...
How is gene therapy changing lives?
Gene replacement therapy. It’s a game changer when it comes to treating life-threatening illnesses. It can replace disease-causing genes with healthy genes, knock out a gene that’s not working right, or add a new gene to the body to help fight disease.
What is one goal of gene therapy?
What is the goal of gene therapy? Introduction of DNA into cells of patient to improve their health by correction of mutant phenotype. Which type of cells does gene therapy target? Deliver of normal gene into appropriate SOMATIC cells. Not desirable to deliver to germline cells (might cause new mutation that would be passed on)
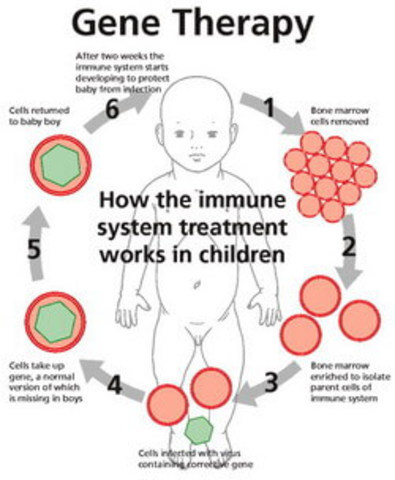
What are some barriers involved in gene therapy?
However, efficacy and safety concerns, immune system responses, laborious approaches for developing and manufacturing, unknown gene-therapy drug interactions with the host and the high cost of drugs/products, are significant barriers in gene therapy.
What is the central challenge for successful gene therapy?
In gene therapy, a normal gene is inserted into the genome to replace an abnormal gene responsible for causing a certain disease. Of the various challenges involved in the process, one of the most significant is the difficulty in releasing the gene into the stem cell.
What are pros and cons of gene therapy?
Gene therapy can be life-saving for some people with specific medical conditions, but it's expensive and can cause side effects. The development of gene therapy is highly regulated by the FDA and National Institute of Health....ConsExpensive. ... Experimental. ... Potentially dangerous. ... Ethical issues. ... May cause infection.
Why is gene therapy so expensive?
One of the reasons gene therapy is currently expensive in terms of research and resource is a lack of a standardised platform to create effective products through. Currently, it is not possible to guarantee that the delivery vector will target the right cell, or that the body will respond as it should. In some previous trials, it has created unwanted immune system reactions, when the vector is detected as a threat or has led to side effects when the ‘wrong’ cells are targeted.
How does gene therapy work?
Gene therapy, in the simplest terms, is the process of delivering genetic material into cells, to account for an abnormality that has given rise to a particular genetic condition. This is delivered through a vector, a modified virus which ‘infects’ the cell to integrate the genetic material. It is considered one of the most important emerging ...
How many gene therapies have been approved?
Whilst there have been thousands of gene therapies trialled, as of 2019, approximately 20 were approved for use – and only 11 within Europe.
How much does Zolgensma cost?
Currently, it is incredibly expensive, costing anywhere from hundreds of thousands of dollars for a single dose, all the way into the millions for certain products, for example, Zolgensma, which treats spinal muscular atrophy and costs £1.78 million for one dose.
Why is genetic therapy important?
It is considered one of the most important emerging fields within advanced therapies, given what it represents for those with life-threatening conditions. Rather than simply treating the symptoms of genetic disorders, it can completely cure them.
When was gene therapy first used?
The first successful gene therapy trial was performed in 1990, to treat severe combined immunodeficiency (SCID), an extremely rare genetic disorder that typically affects one in 35,000 infants in the UK. It reversed the condition in a four-year-old girl.
Is gene therapy a pressure?
Another pressure facing the field of gene therapy, particularly within the UK, is access to training, talent and a skilled workforce. Even over thirty years in, it is very much an emerging field. The Cell and Gene Therapy Catapult, an independent body established by the government to advance the UK’s expertise and commercialisation within the industry, produced a report in 2019 that pointed to a potentially stark skills gap in future years.
How are technological advances helping gene therapy?
Technological advancements are providing a way for gene therapies to deliver long-term benefits to patients with a single dose. This dynamic is expected to introduce a number of issues that will challenge the existing biopharma model, given short-lived demand curves and misalignments between short-term costs of gene therapy and long-term benefits to patients. Addressing the strategic choices behind these challenges will be critical for the sustainability of gene therapy business models.
What is gene therapy?
After three decades of hopes tempered by setbacks, gene therapy (the process of transferring exogenous protein-coding nucleic acids into cells to ameliorate a disease state through restoration or augmentation of host gene function) is poised to make curative 1 therapies a routine approach for managing diseases.
Why are payers reluctant to pay for gene therapy?
This is partly due to the fact that curative therapies do not yet have a long-term track record of sustained efficacy. Under one-time payment models, there is a profound misalignment of short-term costs of gene therapy that would be borne by payers and long-term benefits accrued by patients. Payers are often hesitant to entertain one-time payments that are more than threefold to fivefold the cost of the existing standard of care. Furthermore, they are concerned with having to pay up front for a treatment that would provide benefits to a patient beyond his or her stay on their plan (typically less than five years).
What is Luxturna gene therapy?
At the end of 2017, Spark Therapeutics’ Luxturna, indicated for the treatment of an inherited form of vision loss, became the first in vivo gene therapy approved in the U.S. Further, gene therapy assets from AveXis for spinal muscular atrophy, BioMarin for hemophilia A, and Nightstar for choroideremia, among others, ...
Why is gene therapy important?
This momentum, coupled with scientific, clinical and manufacturing advances, suggests gene therapy will play an important role in managing diseases driven by specific genetic mutations. However, this new treatment paradigm will challenge biopharmaceutical companies to evolve their traditional business models to better serve patients, providers and payers with this complex, novel therapeutic model.
What is the innovation in gene therapy?
Innovation in gene therapy brings the potential for transforming patient care and obviating the need for chronic therapy through single-dose cures. Despite the potential long-term benefits of this new therapeutic modality, gene therapy companies face a number of underappreciated challenges.
How are biotech companies focused on gene therapy?
Most biotech companies focused on gene therapy are formed around a specific technology platform (e.g., novel viral capsid that could have preferential uptake in an organ system). Most then proceed to de-risk the technology as quickly as possible by applying it to low-hanging-fruit indications. However, the main common characteristic across selected indications is often the underlying technology platform that gave rise to the gene therapy candidates. This concentrates risk on the technology platform and exposes the company to a negative event that has a deleterious effect across the whole portfolio. Early-stage gene therapy companies often do not have a choice regarding this risk; however, once the founding technology begins to have traction, executives will often pursue adjacent or orthogonal platforms that diversify risk and maximize opportunities for the company.
What are the challenges of gene therapy?
The first of several significant challenges that have emerged for gene therapy has been safety. Ensuring appropriate delivery of the correct replacement gene to the necessary cells without excessive toxicity. Early on in the field, the death of teenager Jesse Gelsinger in 1999 brought research and investment in gene therapy to a near halt for years.
What is gene therapy?
Gene therapy holds great promise for treating inherited disorders, cancers and other diseases. This is an exciting time, but much remains to be done to address the current hurdles associated with safety and durability to expand the field.
Why are biological drugs limited?
All biological drugs are limited by their ability to provoke the body’s defences, reducing their effectiveness.
How many gene therapies will be approved by 2025?
The FDA predicts 10 to 20 annual cell and gene therapy drug approvals by 2025. 1 Importantly, the research that has generated these approved therapies has taught us about designing safe and effective vectors, ...
What is the context in which APCs encounter antigens?
The context in which APCs encounter antigen influences the outcome of the immune response. For example, vaccines contain immune stimulating molecules that activate APCs to induce effector T cells which help co-ordinate the production of antibodies.
What are the components of a cell therapy?
These therapies, which are derived from living cells and may include protein, enzyme, antibody, antibody-drug conjugates (ADC), gene and cellular components, are of significant value to healthcare considering their ability to treat a range of different diseases.
Is biologics based on gene therapy science fiction?
Most recently, biologics based on cellular and gene therapies have gone from science fiction to true medicines, making it possible to treat disorders where no other treatment options exist for patients .
What is gene therapy?
Thus, gene therapy is understood as the ability of genetic improvement through the correction of altered (mutated) genes or site- specific modifications that target therapeutic ...
What is the ability to make site specific modifications to the human genome?
Thus, gene therapy is understood as the ability of genetic improvement through the correction of altered (mutated) genes or site-spec ….
Why are viruses investigated?
The viruses are more often investigated due to their excellence of invading cells and inserting their genetic material. However, there is great concern regarding exacerbated immune responses and genome manipulation, especially in germ line cells.
What are the challenges faced by gene therapy?
Other challenges faced in the field of gene therapy include: Most gene therapies are short-lived meaning patients need to undergo multiple treatments. Therapeutic DNA needs to be functional in the long-term and the cells containing it need to be long-lived and stable if the therapy is going to provide a permanent cure.
What are the problems with gene therapy?
One of the main issues is the lack of knowledge about the long-term effects of the therapy and the field is fraught with ethical issues.
What are the most common diseases caused by mutations in genes?
Disorders arising from one gene mutation are the most promising candidates for gene therapy but a lot of the most common disorders such as diabetes, heart disease and arthritis are caused by a combination of altered genes making them particularly difficult to treat.
What is the risk of a tumor forming?
There is a risk of inducing tumor growth, a concept referred to as insertional mutagenesis; if the inserted DNA is incorrectly placed, such as in a tumor suppressor gene, then a tumour may form.
Can a person's gene be transferred to gametes?
In other words, if a person has undergone gene therapy whereby the DNA content of their body cells has been modified, there should be no way that the inserted gene can be transferred to the gametes.
Can the immune system respond to gene therapy?
The body's immune system can respond to the modified vectors and disrupt the effectiveness of gene therapy. The immune system's recognition of foreign bodies also means repeated therapy can become problematic.
What is the next critical test of gene therapy?
The next critical test of the gene therapy era will likely come in hemophilia. Multiple companies are racing to market with candidates for hemophilia A, with BioMarin Pharmaceutical the furthest along.
How many gene therapy products will be approved by 2025?
The Food and Drug Administration foresees approving 10 to 20 cell and gene therapy products each year by 2025. Not all of those products, though, are guaranteed to have the compelling efficacy that make Luxturna and Zolgensma relatively easy choices for insurers to cover. The next critical test of the gene therapy era will likely come in hemophilia.
How much does Zolgensma cost?
Novartis priced Zolgensma at $2.1 million, making it the most expensive drug ever brought to market. The Swiss pharma intends for that cost to be spread over multi-year payment plans, the result of lengthy discussions with payers.
Why is the FDA looking for ways to streamline oversight?
If there's a level of similarity, the FDA is looking for ways to streamline oversight so each tiny tweak won't require a different drug application. "If you treated those each as individual drugs, people are never going to get the therapies because you can't expect anyone to put in the effort," he said.
What would happen if there were more uncertain mechanisms of action?
More uncertain mechanisms of action would add back in the "biological" risk of many current experimental drugs, which are often supported by hazy notions of how they work.
Is Zolgensma a regulatory approval?
A landmark regulatory approval has vaulted the cell and gene therapy space to the front of the drug industry's mind. But speedy progress has also brought forward significant challenges. Approved for infants with spinal muscular atrophy, Zolgensma is a stunning advancement for a disease that in its most severe form is fatal before age two.
Who is the director of the FDA's Center for Biologics Evaluation and Research?
Peter Marks , the director of the FDA's Center for Biologics Evaluation and Research, noted gene therapy will face additional challenges in addressing diseases spurred by multiple genes rather than the single gene defects that treatments like Luxturna and Zolgensma target.
What are the two challenges that the gene therapy industry faces?
Regardless of the method, scalability and manufacturability are the two closely related challenges the industry faces, especially if gene therapies are to fulfill their clinical potential.
Why is biological activity retained?
The requirement for biological activity to be retained limits the use of harsh purification methods and adds a special sensitivity that potentially harmful or adventitious agents cannot be introduced through the raw materials supply chain. This is an area that greatly benefits from a close partnership between manufacturers and their raw materials suppliers, to better understand the requirements for cGMP materials and begin meeting them early in the therapy development and manufacturing process.
When will Roche release DMD 3?
The licensing agreement between Roche and Sarepta Therapeutics at the end of 2019 to launch and commercialize a gene therapy addressing Duchenne muscular dystrophy (DMD) 3 only solidifies big pharma’s interest in this growing space. This will bring the sophistication of label and geographic expansion strategies to bear on these therapies in 2020 and beyond.
Who is Ger Brophy?
Ger Brophy, PhD, executive vice president of biopharma production at Avantor, offers insights on how the biopharma industry can evolve its processes through lessons learned from the manufacturing of monoclonal antibodies, supplier collaboration, and other production activities.
Is the industry unable to produce enough gene therapy?
If you look at the gene therapy viral vector levels, the magnitude of the therapy being delivered to the patient in increments from E6 to E16 shows that the industry is simply unable to produce enough to satisfy growing demand.
Is Brophy making progress?
Brophy: Genuine progress is being made in the longstanding battle to effectively treat and control disease, as evidenced by some of the first regulatory approvals for new therapies of their kind and the mergers and acquisitions (M&A) activity within the pharmaceutical industry since 2019.