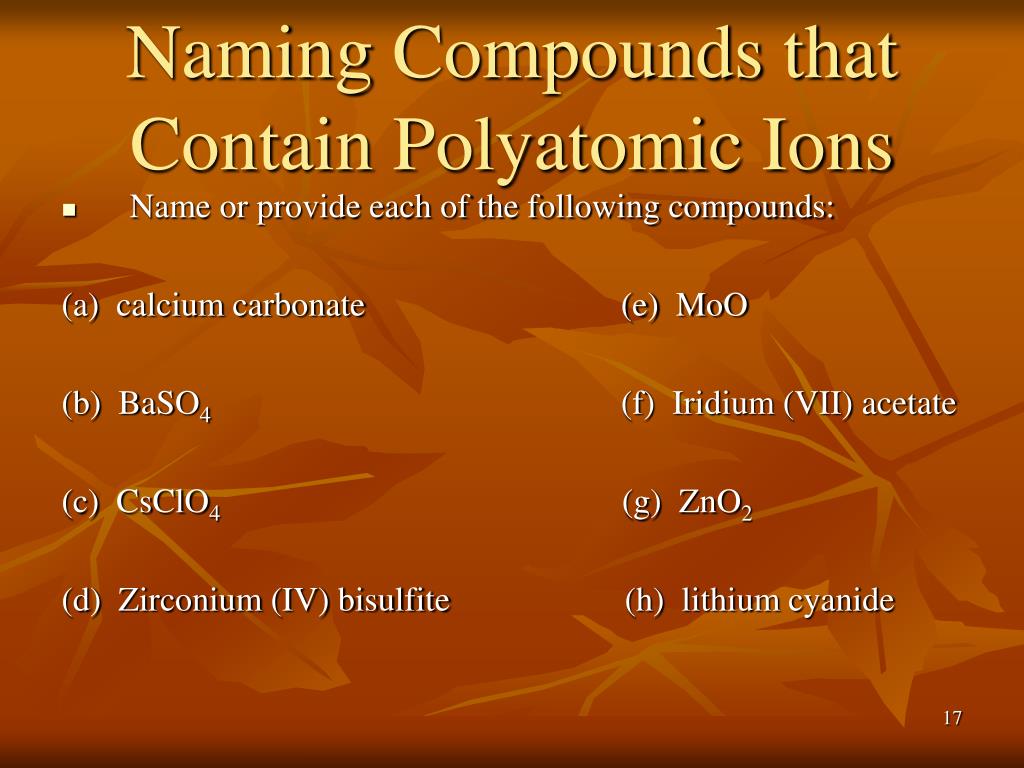
What are 3 examples of polyatomic ions?
Well-known examples of such polyatomic ions are the sulfate ion (SO42–), the hydroxide ion (OH–), the hydronium ion (H3O+), and the ammonium ion (NH4+).
What are 5 examples of polyatomic ions?
Examples of Common Polyatomic IonsAcetate – C2H3O2–Bicarbonate (or hydrogen carbonate) – HCO3–Bisulfate (or hydrogen sulfate) – HSO4–Hypochlorite – ClO–Chlorate – ClO3–Chlorite – ClO2–Cyanide – CN-Hydroxide – OH-More items...
Which compound is ionic and contains a polyatomic ion?
Because these ions contain more than one atom, they are called polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. For example, the nitrate ion has one nitrogen atom and three oxygen atoms with an overall charge of 1−....5.7: Ionic Compounds Containing Polyatomic Ions.NameFormulasulfate ionSO42−9 more rows•Feb 1, 2022
How do you tell if a compound has a polyatomic ion?
0:015:14What's a polyatomic ion? - YouTubeYouTubeStart of suggested clipEnd of suggested clipWell a polyatomic ion is a group of atoms. That has a charge. Here's what I mean you probablyMoreWell a polyatomic ion is a group of atoms. That has a charge. Here's what I mean you probably already know that individual atoms can have a charge they can form ions so we can get things like na plus
What is the most common polyatomic ion?
However, this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. These polyatomic ions are extremely common in chemistry and thus it is important to be able to both recognize and name them....Table of Polyatomic Ions.NameFormulathiosulfateS2O3 2−oxalateC2O4 2−hydroxideOH−6 more rows
Is water a polyatomic ion?
definition. …than two atoms are termed polyatomic molecules, e.g., carbon dioxide (CO2) and water (H2O).
Which compounds do not contain a polyatomic ion?
1 Answersulfate: SO2−4.phosphite: PO3−3.ammonium: NH+4.
Which of the following is polyatomic ion?
Phosphate is polyatomic as it consists of 1P and 4 Oxygen atoms.
What are the 3 types of compounds?
Atoms of more than one type of element can bond together and form compounds. The three main types of bonds are ionic, covalent, and metallic. These distinct types of chemical bonds form distinct types of substances.
Is NH4 a polyatomic ion?
NH4 is the positive polyatomic Ammonium. It is one of the few positive polyatomic ions.
Is Na+ a polyatomic ion?
compounds, except that we use the name of the polyatomic ion whenever it occurs. For example, NaNO2 is named according to its cation, Na+ (sodium), and its polyatomic anion, N03 (nitrite). Its full name is sodium nitrite.
Can covalent compounds have polyatomic ions?
Polyatomic ions are covalent compounds that have an overall charge. In the ammonium ion, NH4 , nitrogen is covalently bonded (shares electrons)to four separate hydrogen atoms.
What are polyatomic ions give two examples?
A polyatomic ion is a group of atoms carrying a charge (positive or negative). For example, nitrate ion (NO−3), hydroxide ion (OH - ).
Is NH4+ a polyatomic ion?
NH4 is the positive polyatomic Ammonium. It is one of the few positive polyatomic ions.
Which ion is a polyatomic ion?
Because these ions contain more than one atom, they are called polyatomic ions. Polyatomic ions have characteristic formulas, names, and charges that should be memorized. For example, NO3− is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1− charge....Polyatomic Ions.NameFormulasulfite ionSO32−16 more rows•Sep 23, 2021
Is Na+ a polyatomic ion?
compounds, except that we use the name of the polyatomic ion whenever it occurs. For example, NaNO2 is named according to its cation, Na+ (sodium), and its polyatomic anion, N03 (nitrite). Its full name is sodium nitrite.
What are 3 examples of polyatomic ions?
Examples of polyatomic ions are Acetate (CH 3 COO – ), Phosphate (PO 4 3- ), Permanganate (MnO 4 – ) , Oxalate (C 2 O 4 2- ) etc.
What is a polyatomic element?
The is made up of two or more atoms, it can be referred to as a polyatomic ion or a molecular ion. Depending on the charge it may be classified a...
Is phosphate a polyatomic ion?
Phosphate (PO 4 3- ) is a polyatomic ion carrying a negative charge. The phosphate ion made more than one atom.
What are monatomic ions called?
The ion is made up of only one type of atom each holding some net charge, positive or negative, it can be referred to as a monatomic ion.
Is Mg 2+ a polyatomic ion?
No Mg 2+ ion is a monatomic ion, it is made up of only one Mg atom.
What is a polyatomic ion?
Polyatomic ions are covalently bonded groups of atoms and having a positive or negative charge caused by the formation of an ionic bond with another ion. Compounds formed from such a combination of ions are polyatomic ionic compounds.
Which polyatomic ions contain oxygen?
The sulfate receives the two electrons to become SO 4-2. Polyatomic Ion NH 4+ or Ammonium: In most polyatomic ions, it contains oxygen and a negatively charged anions. Ammonium is one of the few positively charged polyatomic ions and doesn’t contain oxygen.
How to find the total number of protons in a hydroxide ion?
Ans: We can calculate the total number of protons in a hydroxide ion by adding up the number of protons in one hydrogen atom and one oxygen atom:
Why do monatomic ions have a net charge?
The ion has a net charge over it because the total number of electrons is not balanced by the total number of protons in the nucleus.
Why do polyatomic ions behave differently?
This is because there are two or more atoms present in the polyatomic ion.
What are some examples of monatomic ions?
Thus it is different from monatomic ions, which contain only one atom. Examples of monatomic ions include Na+ and Cl- etc. This article will give details of polyatomic ions and their examples.
What type of ions have a -2 charge?
Polyatomic ions with a -2 charge are also common.
What is a polyatomic ion?
First, let’s define a polyatomic ion. A polyatomic ion is a group of atoms covalently bonded together with a charge that affects the whole polyatomic unit. Some examples of polyatomic ions are in the following table. Make sure you memorize the names, formulas, and charges of each of the ions in this table.
How many polyatomic ions are in chlorine?
Note the halides, I, Cl, and Br, when combined with oxygen, can form four polyatomic ions. For Chlorine, the two additional ions are perchlorate ion, with four oxygens, and hypochlorite ion with only one oxygen. Please refer to the table for the names and formulas of the ions.
What is the parenthesis for ammonium carbonate?
For compounds that contain more than one polyatomic unit, use parenthesis. If we combine ammonium ion, (NH 4+) and carbonate ion (CO 32-) we have ammonium carbonate, (NH 4) 2 CO 3. Two ammonium ions are required for each carbonate ion.
Is phosphoric acid an ous or ic?
and phosphoric acid is formed. The acid must be neutral before naming it with the “ous” or “ic” suffix. More about naming acids in the next study guide.
What is polyatomic ion?
Polyatomic ions are ions made up of more than one atomic element. This example problem demonstrates how to predict the molecular formulas of several compounds involving polyatomic ions.
When writing a formula for an ionic compound, what is the first ion?
When you write the formula for an ionic compound, remember that the positive ion is always listed first. When there are two or more polyatomic ions in a formula, enclose the polyatomic ion in parentheses.
What is the rule for a polyatomic ion?
Rule 1. The positive ion (cation) is written first in the name; the negative ion (anion) is written second in the name. Rule 2. When the formula unit contains two or more of the same polyatomic ion, ...
What are the rules for naming ionic compounds?
Rules for Naming Ionic Compounds Containing Polyatomic Ions. Polyatomic ions are ions which consist of more than one atom. For example, nitrate ion, NO 3-, contains one nitrogen atom and three oxygen atoms. The atoms in a polyatomic ion are usually covalently bonded to one another, and therefore stay together as a single, charged unit.
How to name an anion?
Rule 4. If the anion is a monatomic ion, the anion is named by adding the suffix -ideto the root of the element name (e.g., iodine = I, "iodide" = I-; sulfur = S, "sulfide" = S2-).
Which ion is written first in the name?
Rule 1. The positive ion (cation) is written first in the name; the negative ion (anion) is written second in the name.