
What did Thomson think of atoms?
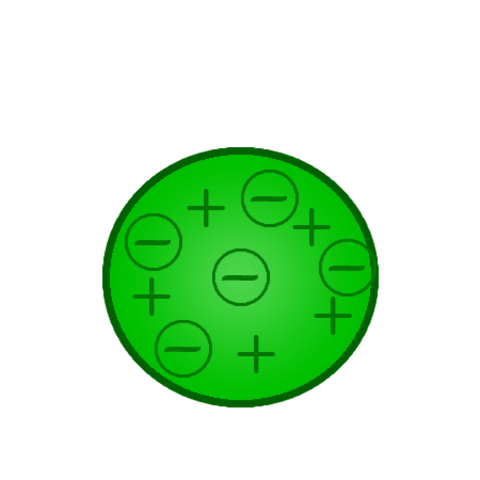
After Eugen Goldstein's 1886 discovery that atoms had positive charges, Thomson imagined that atoms looked like pieces of raisin bread, a structure in which clumps of small, negatively charged electrons (the "raisins") were scattered inside a smear of positive charges.
What is the name of the model that Thomson used to describe the atoms in the pudding?
Lived from: December 18, 1856 - August 30, 1940. Thomson’s model was known as the "Plum Pudding Model” (or "Raisin Bread Model.") As each atom was a sphere filled with a positively charged fluid, known as the “pudding”. Scattered in this fluid were negatively charged electrons, these were the “plums” in the pudding.
Why do positive fluids hold electrons in the atom?
Thomson suggested that the positive fluid held the negatively charged electrons in the atom because of its electrical forces.
Who discovered the electron?
In 1897, J. J. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's work suggested that the atom was not an "indivisible" particle as John Dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific ...
What would happen if an electric current was passed through a vacuum tube?
For years scientists had known that if an electric current was passed through a vacuum tube, a stream of glowing material could be seen; however, no one could explain why. Thomson found that the mysterious glowing stream would bend toward a positively charged electric plate.
What did Thomson propose to explain the overall neutral charge of the atom?
To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge.
Why did Thomson believe their experiments were flawed?
Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because their tubes contained too much gas. Thomson constructed a Crookes tube with a better vacuum.
How did Thomson find the mass to charge ratio of cathode rays?
In his classic experiment, Thomson measured the mass-to-charge ratio of the cathode rays by measuring how much they were deflected by a magnetic field and comparing this with the electric deflection. He used the same apparatus as in his previous experiment, but placed the discharge tube between the poles of a large electromagnet. He found that the mass-to-charge ratio was over a thousand times lower than that of a hydrogen ion (H + ), suggesting either that the particles were very light and/or very highly charged. Significantly, the rays from every cathode yielded the same mass-to-charge ratio. This is in contrast to anode rays (now known to arise from positive ions emitted by the anode), where the mass-to-charge ratio varies from anode-to-anode. Thomson himself remained critical of what his work established, in his Nobel Prize acceptance speech referring to "corpuscles" rather than "electrons".
How did Thomson make the Crookes tube?
Thomson constructed a Crookes tube with a better vacuum. At the start of the tube was the cathode from which the rays projected. The rays were sharpened to a beam by two metal slits – the first of these slits doubled as the anode, the second was connected to the earth.
How did Thomson find the path of cathode rays?
Thomson detected their path by the fluorescence on a squared screen in the jar.
What is the Thomson unit?
In 1991, the thomson (symbol: Th) was proposed as a unit to measure mass-to-charge ratio in mass spectrometry in his honour. J J Thomson Avenue, on the University of Cambridge 's West Cambridge site, is named after Thomson.
What was William Thomson's work on the motion of vortex rings?
Thomson's prize-winning master's work, Treatise on the motion of vortex rings, shows his early interest in atomic structure. In it, Thomson mathematically described the motions of William Thomson 's vortex theory of atoms.
J.J. Thomson Biographical Data
Thomson Atomic Theory
- Thomson's discovery of the electron completely changed the way people viewed atoms. Up until the end of the 19th century, atoms were thought to be tiny solid spheres. In 1903, Thomson proposed a model of the atom consisting of positive and negative charges, present in equal amounts so that an atom would be electrically neutral. He proposed the atom was a sphere, but …
Interesting Facts About J.J. Thomson
- Prior to Thomson's discovery of electrons, scientists believed the atom was the smallest fundamental unit of matter.
- Thomson called the particle he discovered 'corpuscles' rather than electrons.
- Thomson's master's work, Treatise on the motion of vortex rings, provides a mathematical description of William Thomson's vortex theory of atoms. He was awarded the Adams Prize i…
- Prior to Thomson's discovery of electrons, scientists believed the atom was the smallest fundamental unit of matter.
- Thomson called the particle he discovered 'corpuscles' rather than electrons.
- Thomson's master's work, Treatise on the motion of vortex rings, provides a mathematical description of William Thomson's vortex theory of atoms. He was awarded the Adams Prize in 1884.
- Thomson discovered the natural radioactivity of potassium in 1905.