What does all matter consists of?
Explain that all matter on Earth exists in the form of a solid, liquid, or gas, and that solids, liquids, and gases are all made of extremely tiny particles called atoms and molecules.
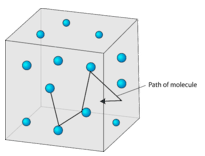
What 3 things does the kinetic theory of matter state?
The simplest kinetic model is based on the assumptions that: (1) the gas is composed of a large number of identical molecules moving in random directions, separated by distances that are large compared with their size; (2) the molecules undergo perfectly elastic collisions (no energy loss) with each other and with the ...
What are the main points of the kinetic theory of matter?
The kinetic theory of matter states that all matter is made of small particles that are in random motion and that have space between them. This means that no matter what phase matter is in, it is made of separate, moving particles.
What state of matter is kinetic?
Moving Matter Any matter that is moving has energy just because it's moving. The energy of moving matter is called kinetic energy. Scientists think that the particles of all matter are in constant motion. In other words, the particles of matter have kinetic energy.
What are the 3 assumptions of the kinetic-molecular theory?
Assumptions of the kinetic-molecular theory include the following: Gas particles are in constant, random motion. The volume of gas particles is negligible in comparison to the volume of the container. There are no attractive forces between gas particles.
What does the kinetic theory of matter state quizlet?
kinetic theory states that all matter consists of tiny particles that are in constant motion and gas pressure is the result of simultaneous collisions of billions of rapidly moving particles in a gas with an object.
The Four Phases of Matter
Matter is anything that has mass and volume. That is, matter is anything that requires energy to accelerate or change its motion and which also takes up space. Matter can come in many forms, but there are four main phases or states of matter: solid, liquid, gas, and plasma.
Kinetic Energy
Kinetic energy is the energy of motion. When matter is in motion, it can be said to possess kinetic energy. All things on Earth technically possess kinetic energy relative to the rest of the universe.
The Kinetic Theory of Matter
The simple kinetic theory definition is the explanation for how or why matter comes in many forms or states of matter, and what makes these states different, and the changes between them possible.

Four Phases of Matter
- Solids, liquids, gases, and plasmas: these are the four phases of matter, which are simply the many forms that matter can take. It’s interesting to note that many compounds can exist in multiple phases. Consider water: it can exist as a solid (ice), a liquid (liquid water), or a gas (wat…
Understanding Behaviour of Matter
- Matter is a substance that occupies space and hasvolume. The matter is made up of atoms and molecules. The state of matter is determined on the basis of the arrangement of its molecules. Temperature, pressure, mass and volume are also the main factors that determine the state of matter. The state of matter may change if the temperature of the surrounding is increased. The …
Illustration: Water
- Let’s take the case of water. The water molecules in their solid-state (ice) have relatively little energy and cannot move away from each other. The molecules are arranged in a regular arrangement known as a lattice. The energy of the molecules in ice increases as it is heated. This means that certain water molecules are able to overcome the intermolecular interactions that ke…
Sample Questions
- Question 1: Why does an egg sinks in salty water but floats in a normal one? Justify? Answer: Question 2: Why was Dalton’s theory a success? Answer: Question 3: Why the energy levels of substances are different from each other? Answer: Question 4: What is the study of crystals called? Define crystal. Answer: Question 5: Write down the formula of the perfect gas equation a…