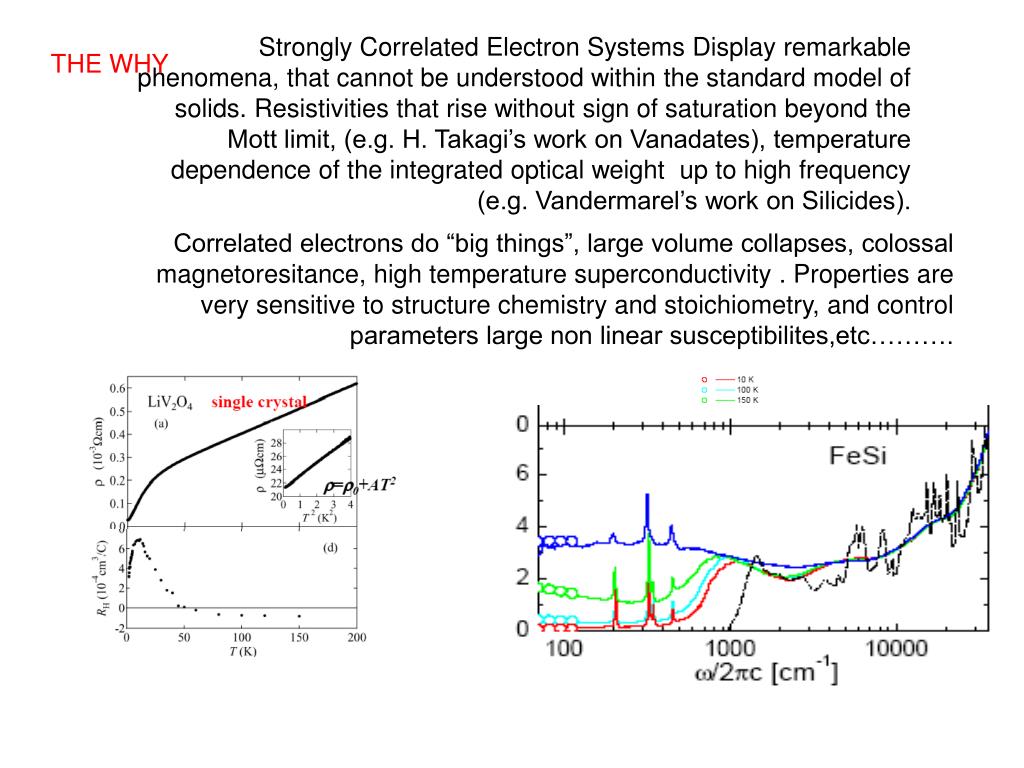
metallic bond The chemical bonding that holds the atoms of a metal together. Metallic bonds are formed from the attraction between mobile electrons and fixed, positively charged metallic atoms.
Full Answer
What is meant by metallic bond?
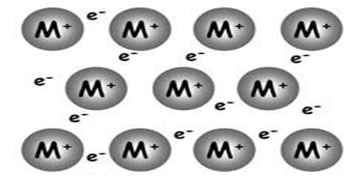
What is a Metallic Bond? ‘Metallic bond’ is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions.
What are facts for metallic bonding?
Metallic bonding is mostly non-polar, because even in alloys there is little difference among the electronegativities of the atoms participating in the bonding interaction (and, in pure elemental metals, none at all). Thus, metallic bonding is an extremely delocalized communal form of covalent bonding.
What is the definition of metallic bonding?
‘Metallic bond’ is a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions. Metallic bonding is a type of chemical bonding and is responsible for several characteristic properties of metals such as their shiny lustre, their malleability, and their conductivities for heat and electricity.
What are some examples of metallic bonding?
- Links between silver (Ag) atoms.
- Bonds between gold (Au) atoms.
- Bonds between cadmium (Cd) atoms.
- Bonds between iron (Fe) atoms.
- Bonds between nickel (Ni) atoms.
- Bonds between zinc (Zn) atoms.
- Bonds between copper (Cu) atoms.
- Bonds between platinum (Pt) atoms.
- Bonds between aluminum atoms (Al).
- Bonds between gallium (Ga) atoms.

What are metallic bonds easy definition?
metallic bond, force that holds atoms together in a metallic substance. Such a solid consists of closely packed atoms. In most cases, the outermost electron shell of each of the metal atoms overlaps with a large number of neighbouring atoms.
What makes a metallic bond?
Metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. Mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Because metals are solid, their atoms are tightly packed in a regular arrangement.
What is a metallic bond answer?
The short answer: metallic bonding is a type of chemical bonding between two or more metal atoms, which arises from the attraction between positively charged metal nuclei and their delocalized valence electrons.
How do you know if a bond is metallic?
Whereas ionic bonds join metals to non-metals, metallic bonding joins a bulk of metal atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms.
What is a metallic bond example?
Some metallic bond examples include magnesium, sodium and aluminum. Metallic bonding causes characteristics or traits that are typical of metals such as malleability, ductility, thermal and electrical conductivity, opacity and luster.
What is a metallic bond quizlet?
A metallic bond is the force of attraction between a positively charged metal ion and the valence electrons it shares with other ions of the metal. The electrons move freely around the positive ions, which form a lattice-like structure. With freely moving electrons, metals are good conductors of electricity.
Which of the following best describes a metallic bond?
Which of the following best describes metallic bonding? Some metal atoms gain electrons while others lose electrons, resulting in the formation of positive and negative charges.
Are metallic bonds ionic?
Summary – Ionic Bonding vs Metallic Bonding The key difference between ionic bonding and metallic bonding is that the ionic bonding takes place between positive and negative ions whereas the metallic bonding takes place between positive ions and electrons.
What properties do metallic bonds have?
Metallic bonds The metallic bond is the force of attraction between these free-moving (delocalised) electrons and positive metal ions . Metallic bonds are strong, so metals can maintain a regular structure and usually have high melting and boiling points. Metals are good conductors of electricity and heat.
What is the difference between metallic and ionic bonds?
An ionic bond is formed when one atom donates valence electrons to another atom. A covalent bond is formed when both the atoms share pairs of valence electrons. A metallic bond is formed between a cloud of free electrons and the positively charges ions in a metal.
Is metallic bond a covalent bond?
Metallic bonding is mostly non-polar, because even in alloys there is little difference among the electronegativities of the atoms participating in the bonding interaction (and, in pure elemental metals, none at all). Thus, metallic bonding is an extremely delocalized communal form of covalent bonding.
What is the difference between covalent and metallic bonding?
Metallic bonding is different from covalent bonding in that in metal bonding all atoms give off their extra electrons and form a sea of electrons, whereas, in covalent bonding, atoms share their electrons locally.
1. What are the properties of metallic bonds?
A metallic bond is a force that is held together within the metallic elements. The presence of metallic bonds gives rise to their properties. Stren...
2. Why are metallic bonds strong?
Due to the metallic bonds present in elements, the atoms are held together tightly. The structure of metals are mostly compact and atoms are arrang...
3. How do atoms bond in metallic bonding?
In a metallic bond, the atoms are held together with a force. Metallic Bond happens when there is a share of valence electrons. For example, if sod...
4. Do metallic bonds conduct electricity?
Metallic bonding is described as the sharing of electrons over a large area as compared to the size of the structure. And hence when these electron...
5. What is the difference between metallic bonding and ionic bonding?
Metallic Bonding is formed when there is a sharing of electrons over an area. whereas ionic bonding is formed when there is the transfer of electro...
Why do metallic bonds occur?
Metallic bonding may be seen as a consequence of a material having many more delocalized energy states than it has delocalized electrons (electron deficiency), so localized unpaired electrons may become delocalized and mobile. The electrons can change energy states and move throughout a lattice in any direction.
How do metallic bonds work?
How Metallic Bonds Work. The outer energy levels of metal atoms (the s and p orbitals) overlap. At least one of the valence electrons participating in a metallic bond is not shared with a neighbor atom, nor is it lost to form an ion.
What type of bond is formed between positively charged atoms?
Updated September 07, 2019. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. In contrast, covalent and ionic bonds form between two discrete atoms. Metallic bonding is the main type of chemical bond that forms between metal atoms.
Why are metals malleable?
Malleability: Metals are often malleable or capable of being molded or pounded into a shape, again because bonds between atoms readily break and reform. The binding force between metals is nondirectional, so drawing or shaping a metal is less likely to fracture it. Electrons in a crystal may be replaced by others.
What type of bond is found between metals?
Metallic bonding is the main type of chemical bond that forms between metal atoms. Metallic bonds are seen in pure metals and alloys and some metalloids. For example, graphene (an allotrope of carbon) exhibits two-dimensional metallic bonding. Metals, even pure ones, can form other types of chemical bonds between their atoms.
Why do electrons in a crystal change?
Further, because the electrons are free to move away from each other, working a metal doesn't force together like-charged ions, which could fracture a crystal through the strong repulsion. Metallic luster: Metals tend to be shiny or display metallic luster.
Why are metals good conductors?
Electrical conductivity: Most metals are excellent electrical conductors because the electrons in the electron sea are free to move and carry charge. Conductive nonmetals (such as graphite), molten ionic compounds, and aqueous ionic compounds conduct electricity for the same reason—electrons are free to move around.
What is the type of chemical bond between atoms in a metallic element?
noun Chemistry. the type of chemical bond between atoms in a metallic element, formed by the valence electrons moving freely through the metal lattice.
What is the chemical bonding that holds the atoms of a metal together?
metallic bond . The chemical bonding that holds the atoms of a metal together. Metallic bonds are formed from the attraction between mobile electrons and fixed, positively charged metallic atoms. Whereas most chemical bonds are localized between specific neighboring atoms, metallic bonds extend over the entire molecular structure.
Is it ridiculous to marry yourself?
Marriage is a bond and a commitment—marrying yourself is ridiculous because you are already married to yourself. He was released within the hour without a bond on his own recognizance. "Garnache," came the other's crisp, metallic voice, and the name had a sound as of an oath on his lips.
What is the bond between metals?
Therefore, free electrons act as the cohesive force which holds the metal atoms together and forms a metallic bond. The bond produced due to the combination of the electrostatic force ...
What is the bond between the electrons and the nuclei of a metal called?
The bond produced due to the combination of the electrostatic force of attraction between the electrons and the positive nuclei of metal atoms is called a metallic bond.
How do valence electrons move?
The valence electrons being very light, can move in the electron sea from one position to the other. The kernels being heavy, have comparatively negligible movement. The force of attraction between the positively charged kernels and the valence electron gives rise to the formation of metallic bonds.
What are the factors that affect the strength of a metallic bond?
What are the Factors Affecting Metallic Bond? 1 The number of delocalised valence electron: The strength of the metallic bond increases with an increase in the number of delocalized valence electrons. 2 Size of the kernel: The strength of the metallic bond decreases with an increase in the size of the kernel.
Why are metals good conductors of heat and electricity?
Metals have free electrons, which can transfer energy rapidly. Therefore metals are good conductors of heat and electricity. 3. Metals are lustrous due to the absorbs of radiation by the valence electrons of the metal, and they also emit light as a reflection of the original light.
What is the attractive force that holds together atoms, molecules, ions, or a combination of these called
The attractive force which holds together atoms, molecules, ions, or a combination of these is called a chemical bond. These are different types of bonds like a covalent bond, ionic bond, hydrogen bond, metallic bond, etc. In this article, let us understand every thing about metallic bonding, properties, and examples of a substance exhibiting ...
Which type of bonding is the attractive force which comes into existence due to the mutual sharing of electrons between two
Example: Sodium chloride, Magnesium oxide, calcium chloride, etc., show this type of bonding. Covalent bond: The attractive force which comes into existence due to the mutual sharing of electrons between two atoms of similar electronegativity is having a small difference in electronegativities is called a covalent bond.
When does a metallic bond break?
Metallic bond is completely broken when a metal boils, but it only slightly loosens when it melts.
What are the factors that determine the strength of a metallic bond?
The strength of any metallic bond depends upon three factors: 1) The number of electrons that gets delocalized from the metal. 2) The charge of the metallic ion. 3) The size of the metallic ion.
What type of bond is hydrogen and oxygen?
Sometimes atoms like hydrogen and oxygen share electrons to fill their valence shell. This kind of bond is called covalent bond. Sometimes, a large number of metallic atoms come together and many of the electrons in their valence shell is detached and roams around the remaining positive ions, in a way being shared among all of them. ...
Why do metals have free electrons?
The properties of metals in free state is due to the arrangement of electrons in the metallic bond. Since valence electrons are free, delocalized, mobile and not associated with any particular atom, it is possible to explain several properties of metals.
What type of bond is formed when a simple metal donates an electron and gets a positive charge?
This kind of bond is called ionic bond. Sometimes atoms like hydrogen and oxygen share electrons to fill their valence shell.
Why are metals shiny?
Free electrons in the sea of electrons can freely absorb photons and hence metals are opaque looking. Electrons on the surface of the metal can bounce back light at the same frequency at which the light hits its surface, and thus metals appear shiny.
How do electrons move in a metallic bond?
In metallic bonds electrons are free to move about within the confines of the crystals they exist in. This makes it easy for electric charge to move in. If electrons from any outside force are pushed into a metal due to an electric circuit, the electrons can move through the electron sea and come out from the other end of the metal connected to the electric circuit.
Why are metallic bonds used in metal alloys?
Metallic bonds can be used to make metal alloys. Metallic bonds are used via copper wires in a house in order to transfer electricity due to its conductive properties.
What is the chemical bond between metals?
The metallic bond is a unique type of chemical bond found in metal elements. In a sample of metal, the valence electrons detach from the atoms and are free to move throughout the metal. This results in a sea of electrons, or big pool of delocalized or valence electrons. The sea of electrons is negative and the metal atoms have become positive ions.
What happens when electrons move in a metal bond?
The electrons are free to move within a metallic bond and they carry a negative charge. The movement of the electron coupled with its charge results in the conductivity of the compound (conductivity is the movement of charge). 3. The bonds between metal atoms can be easily broken and then formed again.
What is the pool of valence electrons in metal?
All of the atoms in that small piece of metal are sharing a big pool of valence electrons known as a sea of electrons or delocalized electrons. The big pool is like a free-for-all in that any valence electron can move to any atom within the material. {"error":true,"iframe":true}.
What is the attraction between the two parts of a metal?
The sea of electrons is negative and the metal atoms have become positive ions. The attraction between the two parts of the metal is the metallic bond. This special type of bond gives metals unique properties. Metals are malleable and ductile, which means they can be shaped or stretched into thin wires without breaking.
What is the difference between metallic and covalent bonds?
A metallic bond is pretty different from covalent and ionic bonds, but the goal is the same: to achieve a lower energy state. Instead of a bond between just two atoms, a metallic bond is a sharing of electrons between many atoms of a metal element. Take a look at your desk and see if you can find a small piece of metal like a paper clip or a staple.
What happens when two atoms share electrons?
When two atoms share electrons, they form a covalent bond. When one atom takes an electron away from another and the resulting positive and negative ions are attracted to each other, those atoms have formed an ionic bond.
What is metallic bond?
A metallic bond is a chemical bond, in which the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal, so that each electron can interact with many of the fixed atoms. The free electrons shield the positively charged ion cores from ...
What are the properties of metallic bonding?
The free movement or delocalization of bonding electrons leads to classical metallic properties such as luster (surface light reflectivity), electrical and thermal conductivity, ductility, and high tensile strength.
What is the unique feature of metals?
The unique feature of metals as far as their structure is concerned is the presence of charge carriers, specifically electrons. This feature is given by the nature of metallic bond. The electrical and thermal conductivities of metals originate from the fact that their outer electrons are delocalized. References:
What is chemical bonding?
A chemical bond is a lasting attraction between these atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays ...
Is metal a solid or a mixture?
Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminum, copper, chromium, titanium, gold, nickel), and often also nonmetallic elements (e.g., carbon, nitrogen, oxygen) in relatively small amounts. The unique feature of metals as far as their structure is concerned is the presence of charge carriers, ...