What determines the number of atoms in an element?
To calculate the number of atoms in a sample, you need to find how many moles of the element the sample contains. A mole is a unit chemists use. It's equal to Avogadro's number (6.02 X 10 23) of atoms. By definition, the weight of one mole of an element (its molar mass) is equal to its atomic weight in grams.
What will determine the atomic number of an element?
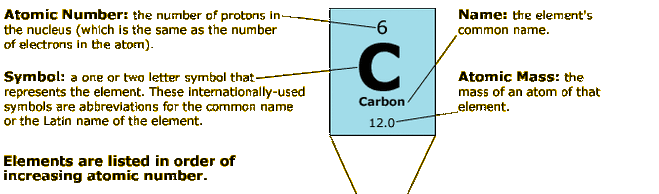
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of [He] 2s 2 2p 2, since its atomic number is 6.
Does each element have its own atomic number?
Each element on the periodic table has its own atomic number. In fact, this number is how you can distinguish one element from another. The atomic number is simply the number of protons in an atom. For this reason, it's sometimes called the proton number. In calculations, it is denoted by the capital letter Z.
Does the atomic number determines the identity of an element?
The number of protons in one atom of an element determines the atom's identity, and the number of electrons determines its electrical charge. The atomic number tells you the number of protons in one atom of an element. The atomic mass of an element is the total number of protons and neutrons in the nucleus of the atom.
What does the atomic number of an element represent Brainly?
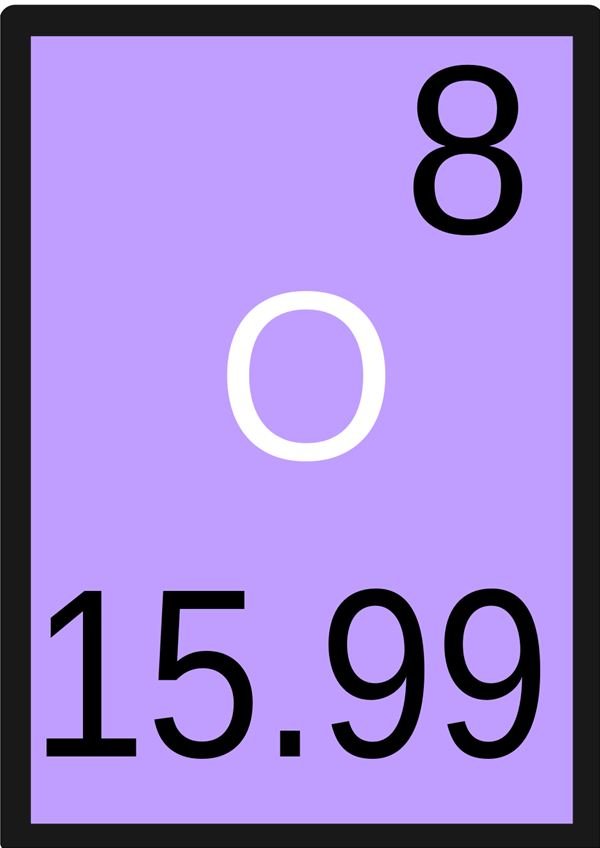
Answer. Answer: Atomic number of an element represents the number of protons in the nucleus. It is also equal to the number of electrons in neutral atom.
What does the atomic number of an element represent what does the mass number of an atom represent?
The mass number tells us the number the sum of nucleons i.e number of protons and neutrons in the nucleus of an atom. The atomic number is the number of protons found in the nucleus of an atom. It is traditionally represented by the symbol Z.
What does the atomic mass unit represent?
The atomic mass unit (AMU or amu) of an element is a measure of its atomic mass. Also known as the dalton (Da) or unified atomic mass unit (u), the AMU expresses both atomic masses and molecular masses. AMU is defined as one-twelfth the mass of an atom of carbon-12 (12C).
Why does atomic number considered to be the fingerprint of elements?
The atomic spectrum of an element is called the finger print because one can identify the element looking at the spectrum. The spectrum of two different elements can not be exactly similar just like the finger print of two different persons.
What does the atomic number tell you?
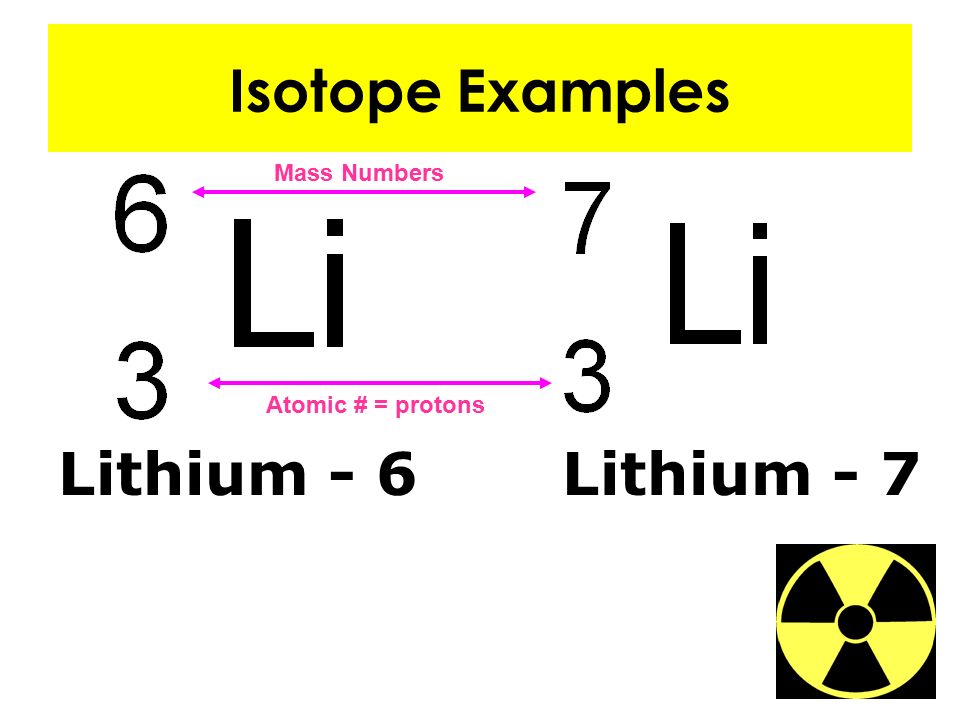
The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.
What 2 things does the atomic number represent?
The number of protons in a nucleus is called the atomic number and always equals the number of electrons in orbit about that nucleus (in a nonionized atom).
What does an atomic symbol represent?
The atomic symbol represents a code to identify an specific element in the Periodic Table of Elements. Atomic symbols normally consist of one or two letters from the alphabet, with the first letter capitalized, but sometimes they can contain three letters when the element has a temporary name.
Is atomic mass and mass number the same?
Atomic Mass and Mass Number Don't Mean the Same Thing There is a difference between the meanings of the chemistry terms atomic mass and mass number. One is the average weight of an element and the other is the total number of nucleons in the atom's nucleus. Atomic mass is also known as atomic weight.
How do you read the atomic mass?
0:552:23Understanding Atomic Number and Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe number that appears below the element symbol is called the atomic mass the mass of an atomMoreThe number that appears below the element symbol is called the atomic mass the mass of an atom depends on the number of protons neutrons.
Why is atomic number more important?
Answer: The atomic number represents the number of the electron in an element. Most of the chemical property of an element depends on electrons in their outermost shell which is called a valency shell. Hence, the atomic number is more important to a chemist than its relative atomic mass.
Why atomic number is important in the synthesis of new elements?
Why the Atomic Number Is Important. The main reason the atomic number is important is because it's how you identify the element of an atom. Another big reason it matters is because the modern periodic table is organized according to increasing atomic number.
What is called atomic number?
atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
What does an element's atomic number represent quizlet?
The atomic number of an atom represents the number of protons located in its nucleus. (Since elements are neutrally charged, the number of protons are equivalent to the number of electrons. Thus, the atomic number also tells the number of electrons.)
What is the difference between the mass number and the atomic number of an atom?
The major difference between atomic number and mass number is that the atomic number states the number of protons present in an atom whereas, the mass number indicates the total number of protons and the number of neutrons present in an atom.
What does the atomic number represent apex?
Atomic number of an element shows the number of protons in the nucleus of that atom.
What is the mass number used for?
The mass number, (also called nucleon number), refers to the total number of protons and neutrons in the nucleus of an atom, and is used to organize the chart of nuclides. Each chemical element has a different number of protons, often with different numbers of neutrons.
Answer
The atomic number represents or stands for the distinct identity of a chemical element. It is usually defined as the number or protons present in an atom of an element, which is also equal to the number of electrons.
New questions in Chemistry
How do weak intermolecular bond strengths affect the melting and boiling point of a substance? The melting point is lower, but the boiling point is hi …
Answer
The atomic number represents or stands for the distinct identity of a chemical element. It is usually defined as the number or protons present in an atom of an element, which is also equal to the number of electrons.
New questions in Physics
Question 3 (Essay Worth 5 points) A train leaves the station heading south on the tracks. It takes the train 5 seconds to reach 50 miles per hour. It …
What does the atomic number tell us about an element?
Every element on the periodic table has an atomic number that tells us how many protons are in the nucleus of an atom of that element . To understand this a little better, let's recall the structure of an atom.
How to find the atomic number of an element?
When we look at the periodic table, we will find an element's atomic number at the top left-hand corner of the box containing its symbol. The elements are arranged by their atomic numbers with the numbers increasing one by one as we move from left to right across each period (or horizontal row). We can see, then, that hydrogen has an atomic number of 1, for it has one proton in its nucleus. It also has one electron. Iron, on the other hand, has an atomic number of 26, so it has twenty-six protons.
How many particles are in an atom?
An atom is made up of three particles, protons, neutrons, and electrons. The protons and neutrons are found in the atom's nucleus, its center, while the electrons are found on the electron shells arranged around the nucleus. Atoms have equal numbers of protons and electrons so that the atom's charge remains balanced. Protons are positively charged, and electrons are negatively charged. Looking at an element's atomic number, therefore, also tells us the number of electrons that element has in each of its atoms.
What is the atomic number of an element?
The atomic number of an element is the number of protons the atom has in its nucleus and, because each element has a different number of protons, this number determines the element’s identity.
Why is the atomic mass of each element on the periodic table a decimal?
This is because the value shown for the atomic mass is actually a weighted average, determined using the proportional amounts of all the isotopes of that element on Earth.
How to find the atomic mass of an element?
So, the atomic mass of an element is calculated by adding together the number of protons and neutrons an atom has. On the periodic table, the atomic mass of elements increases as the atomic number increases, with a few exceptions.
How many forms of each element are there on Earth?
More than one form of each element exists on Earth; these variants are called isotopes and differ from each other only in the number of neutrons each has in its nucleus. When determining the mass of an atom, protons and neutrons are counted, and they have masses of approximately 1 atomic mass unit each. Electrons are negligible in atomic mass ...
Why round the atomic mass to the nearest whole number?
Because there is usually one isotope that is much more common on Earth, rounding the atomic mass to the nearest whole number gives the atomic mass of that isotope, and is called the atomic mass number.
What is the atomic mass of sodium?
For example, on the periodic table, sodium (Na) has an atomic number of 11 and an atomic mass of 22.990. The decimal 22.990 indicates that there are multiple isotopes of sodium, each with a different number of neutrons.
How many neutrons does sodium have?
Thus, the atomic mass is rounded to the nearest whole number, 23. Each atom of sodium therefore has 11 protons and 12 neutrons in its nucleus (11 p + + 12 n o = 23) and 11 electrons orbiting in its shells.
What does the atomic number of an element show?
Explanation: Atomic number of an element shows the number of protons in the nucleus of that atom. Atomic number of an element is always a positive integral value. Answer link.
What does Z mean in chemistry?
And by definition, Z represents the number of MASSIVE, POSITIVELY charged particles, i.e. nuclear protons, that are present in the element's nucleus, and Z thus defines the IDENTITY of the given nucleus.
What are the particles in the nucleus?
Also present in the nucleus are neutrons, massive particles of ZERO charge, and interactions between protons and neutrons at these infinitesimally small nuclear ranges comprise the strong nuclear force, which is strong enuff to overcome the electrostatic force of REPULSION between the like-charged protons.