
What does the IRB IEC evaluate? An IRB/IEC reviews the appropriateness of the clinical trial protocol as well as the risks and benefits to study participants. It ensures that clinical trial participants are exposed to minimal risks in relation to any benefits that might result from the research.
What is an IRB/IEC in clinical trials?
IRBs can also be called independent ethics committees (IECs). An IRB/IEC reviews the appropriateness of the clinical trial protocol as well as the risk and benefits to study participants.
What is an IRB (Institutional Review Board)?
The ICH defines an institutional review board (IRB) as a group formally designated to protect the rights, safety and well-being of humans involved in a clinical trial by reviewing aspects of the trial and approving its start-up. IRBs can also be called independent ethics committees (IECs).
What is the composition of the IRB/IEC?
3.2 Composition, Functions and Operations 3.2.1 The IRB/IEC should consist of a reasonable number of members, who collectively have the qualifications and experience to review and evaluate the science, medical aspects, and ethics of the proposed trial. It is recommended that the IRB/IEC should include: (a) At least five members.
What should the IRB/IEC review when making payments to subjects?
3.1.8 The IRB/IEC should review both the amount and method of payment to subjects to assure that neither presents problems of coercion or undue influence on the trial subjects. Payments to a subject should be prorated and not wholly contingent on completion of the trial by the subject.
Why should IRB/IEC review payment?
What are the responsibilities of an IRB?
How many members should be in an IRB?
How often should IRB review a trial?
Who can ask the IRB to provide its written procedures and membership lists?
Who should vote on IRB review?
How long should IRB keep records?
See 2 more
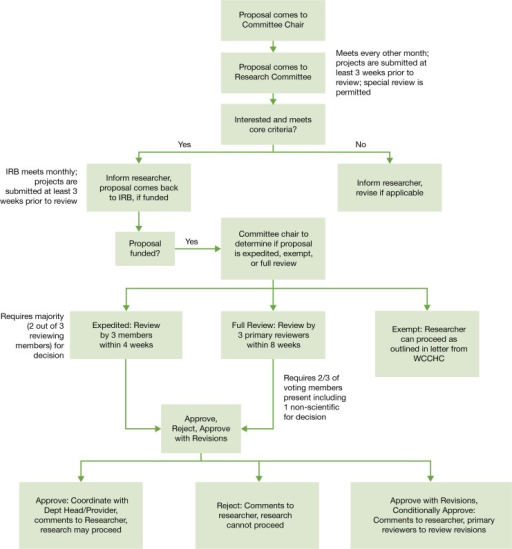
What is IRB evaluation?
Under FDA regulations, an IRB is an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research.
What are the responsibilities of an IRB IEC select all that apply?
What are the Responsibilities of the IRB/IEC? An IRB/IEC should safeguard the rights, safety and well-being of all trial subjects. Special attention should be paid to trials that may include vulnerable subjects.
What is the main difference between IEC and IRB?
What is the difference between an IRB and IEC? Clinical trials conducted in the European Union are held accountable by Independent Ethics Committees (IECs). In the United States, Institutional Review Boards (IRBs) have oversight and must abide by the United States Food and Drug Administration (FDA) regulations.
Which of the following documents should an IRB IEC review to satisfy this responsibility?
The IRB/IEC should obtain the following documents: trial protocol(s)/amendment(s), written informed consent form(s) and consent form updates that the investigator proposes for use in the trial, subject recruitment procedures (e.g. advertisements), written information to be provided to subjects, Investigator's Brochure ...
What are the responsibilities of the IRB?
The IRB is concerned with protecting the welfare, rights, and privacy of human subjects. The IRB has the authority to approve, disapprove, monitor, and require modifications in all research activities that fall within its jurisdiction as specified by both the federal regulations and institutional policy.
Which of the following is a type of IRB IEC review?
IRB must review all projects that meet the definition of research and that involve human subjects prior to any data collection to determine the appropriate level of review, and, as appropriate, approve them. There are three major types of review: Exempt, Expedited, and Full.
What is IRB IEC and regulatory authority?
An IRB/IEC is an ethical committee with the primary motive to review the researches or experiments that are conducted by considering ethical measures or not which is constituted under FBA regulations to safeguard the rights and welfare of research involving human volunteers.
What is IEC in research?
INSTITUTIONAL ETHICS COMMITTEE (IEC) INITIAL REVIEW SUBMISSION FORM FOR ETHICAL CLEARENCE (Form to be filled by the Principal In. Page 1. INSTITUTIONAL ETHICS COMMITTEE (IEC) INITIAL REVIEW SUBMISSION FORM FOR ETHICAL CLEARENCE. (Form to be filled by the Principal Investigator (PI)/Supervisor for submission to IEC)
What needs IRB approval?
FDA regulations generally require IRB review and approval of research involving FDA-regulated products (e.g., investigational drugs, biological products, medical devices and dietary supplements) (21 CFR Part 56).
What are the requirements for the composition of an IRB IEC?
The recommended composition of an IRB includes at least five members, with at least one whose primary area of interest is in a nonscientific area, and at least one member who is independent of the institution or trial site. Nonmembers with expertise in special areas may be invited.
What is the IRB approval process?
The IRB office utilizes an initial pre-review screening process, during which an IRB analyst reviews each submission for completeness and compliance. The analyst may ask the PI to make changes to the submission before it is reviewed by the IRB (pre-review).
What information should be provided to an IRB for review at the initiation of a study choose the best response?
This is Expert Verified Answer C. The study protocol (and amendments), the information to be given to the subject (informed consent, advertisements), the Investigator Brochure (or drug label), any other relevant safety information, and an outline of the qualifications of the investigator.
What does the IRB do quizlet?
Institutional review boards (IRBs) are tasked with reviewing all studies involving human subjects to protect their rights and welfare.
Which of the following is the primary role of an Institutional Review Board IRB quizlet?
Institutional Review Board (IRB) The primary purpose of the IRB is to protect the rights and welfare of human subjects involved in research activities being conducted under its authority.
Which of the following is a function of institutional review boards IRBs quizlet?
What is the function of an Institutional Review Board (IRB)? To review the ethical aspects of a study before it gets underway.
What should be the composition of an IRB IEC?
IRB/IEC Membership At least five members. Members with varying backgrounds. At least one member who represents a non-scientific area (a lay member). At least one member who is not affiliated with the institution or the trial site (an independent member).
IRB/IEC - Same, different or similar?
ICH GCP section 3 is devoted to Institutional Review Board/Independent Ethics Committee (IRB/IEC) which suggests these two bodies are the same. Their definitions in the glossary (1.
8. Essential documents for the conduct of a clinical trial
Essential Documents are those documents which individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced. These documents serve to demonstrate the compliance of the investigator, sponsor and monitor with the standards of Good Clinical Practice and with all applicable regulatory requirements.
ICH GCP - 4. INVESTIGATOR
4.1 Investigator’s Qualifications and Agreements. 4.1.1 The investigator(s) should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial, should meet all the qualifications specified by the applicable regulatory requirement(s), and should provide evidence of such qualifications through up-to-date curriculum vitae and/or other ...
E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1)
E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1) Guidance for Industry . U.S. Department of Health and Human Services . Food and Drug Administration
Investigator Responsibilities and Good Clinical Practice (GCP)
Tool: Training Presentation: Investigator Responsibilities and Good Clinical Practice (GCP) Based on ICH . E6. GCP Guidance (Sections1.24 & 6) and 45
What is the difference between an IRB and IEC?
Clinical trials conduct ed in the European Union are held accountable by Independent Ethics Committees (IECs). In the United States, Institutional Review Boards (IRBs) have oversight and must abide by the United States Food and Drug Administration (FDA) regulations. Countries other than those in the European Union and the United States have individual committees and regulations.
Why are IECs and IRBs so critical?
Independent Ethics Committees and Institutional Review Boards are necessary to monitor all aspects of clinical trials, ensuring that human trial participants’ safety is their top priority. They review each study through neutral eyes, evaluating the risks and benefits throughout every clinical trial process step.
What is an Institutional Review Board (IRB)?
An Institutional Review Board is a formally designated group assigned to monitor and review any medical research involving human subjects. This team has the authority to approve or disapprove research and ask for research modifications to lead to approval. IRBs operate under United States Food and Drug Administration (FDA) regulations.
How are IRBs and IECs formed?
Per the FDA, each IRB/IEC should include five or more members and come from various backgrounds. One of these representatives should come from a non-scientific background and is referred to as a lay member. Another should be an independent member who is not affiliated with the clinical site or medical institution. All members should be competent individuals who are able to understand all aspects of the clinical trial process.
How do IECs / IRBs relate to patient payments?
Each country across the world has its regulations for stipend and per diem payments, patient reimbursements, and tax reporting of income related to clinical trial participation. When conducting a trial at sites in various countries, understanding these unique requirements is hugely time-consuming and overwhelming. Often, countries will make changes to their guidelines, causing the need to quickly pivot to adapt to these updates and adhere to the new rules.
What is the role of IRBs in clinical trials?
Developing a patient-centric and compliant study is a top priority for any clinical team. Understanding the importance of Institutional Review Boards (IRB) and Independent Ethics Committees (IEC) and their role is critical to a successful clinical trial. Before a trial can begin, however, each aspect of the study must first be reviewed and approved by a designated regulatory body. IRBs and IECs check each component of the clinical trial, from protocol development to patient reimbursement and travel support. Below, you will learn about several types of these boards and how Clincierge helps sponsors and CROs keep logistics support and reimbursement compliant to avoid any study delays.
What are the two types of IRBs?
There are two types of IRBs and IECs, local and central. Local committees support individual research institutions and are responsible for reviewing only their clinical trials. Central boards oversee the review of clinical studies for many different organizations.
What is an IRB?
Institutional Review Boards (IRBs) and Protection of Human Subjects in Clinical Trials. Under FDA regulations, an Institutional Review Board is group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, ...
What is the role of an IRB?
In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
Why do IRBs review research protocols?
To accomplish this purpose, IRBs use a group process to review research protocols and related materials (e.g., informed consent documents and investigator brochures) to ensure protection of the rights and welfare of human subjects of research.
What is the role of IRB/IEC?
An IRB/IEC should function in compliance with its own written operating procedures and in full compliance with local regulations and GCPs. It must also maintain written minutes of its meeting and a list of members and their qualifications
Who may request written copies of records maintained by the IRB/IEC?
Investigators, sponsors, and regulatory authorities may request written copies of what records maintained by the IRB/IEC?
Who should notify investigators and institutions of its trial related decisions/opinions and the basis for such decisions/o?
The IRB/IEC should promptly notify investigators and institutions of its trial related decisions/opinions and the basis for such decisions/opinions. It must also provide appeal procedures for such decisions/opinions
Can IRB members vote on study decisions?
Although an IRB/IEC member is not present at a convened meeting, he or she may still vote on a study decision, provided that he/she has fully reviewed the proposal prior to the meeting. True or False
Can an investigator provide information to the IRB?
An investigator may provide information to the IRB/IEC, but should not participate in deliberations or the vote/opinion. True or False
What is the purpose of the IRB?
The purpose of the IRB is to assure that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in a research study.
What is an IRB?
An institutional review board ( IRB ), also known as an independent ethics committee ( IEC ), ethical review board ( ERB ), or research ethics board ( REB ), is a type of committee that applies research ethics by reviewing the methods proposed for research to ensure that they are ethical. Such boards are formally designated to approve (or reject), ...
How many members are needed for an IRB?
For example, the minimum number of members is five, at least one scientist, and at least one non-scientist. The guidance strongly suggests that the IRB contain both men and women, but there is no regulatory requirement for gender balance in the IRB's membership. The full requirements are set out in 21 CFR 56.107.
What is expedited review?
An expedited review may be carried out if the research involves no more than minimal risk to subjects, or where minor changes are being made to previously approved research. The regulations provide a list of research categories that may be reviewed in this manner.
What are the ethical principles of the Belmont Report?
The result of these abuses was the National Research Act of 1974 and the development of the Belmont Report, which outlined the primary ethical principles in human subjects review; these include "respect for persons", "beneficence", and "justice".
What are formal review procedures?
Formal review procedures for institutional human subject studies were originally developed in direct response to research abuses in the 20th century . Among the most notorious of these abuses were the experiments of Nazi physicians, which became a focus of the post-World War II Doctors' Trial, the Tuskegee Syphilis Study, a long-term project conducted between 1932 and 1972 by the U.S. Public Health Service, and numerous human radiation experiments conducted during the Cold War. Other controversial U.S. projects undertaken during this era include the Milgram obedience experiment, the Stanford prison experiment, and Project MKULTRA, a series of classified mind control studies organized by the CIA .
How often should a trial be reviewed?
Ensure that the proposed trial is reviewed within a reasonable time and document opinions and decisions in writing, clearly identifying the trial, the documents reviewed and recorded dates for approvals, required modifications prior to approval, disapproval of a proposed trial, or termination/suspension of any prior approval. Continuing review of ongoing trials is required at intervals appropriate to the degree of risk to human subjects, but at least once per year.
What is the role of IRB/IEC?
An IRB/IEC reviews the appropriateness of the clinical trial protocol as well as the risks and benefits to study participants. It ensures that clinical trial participants are exposed to minimal risk in relation to any benefits that might result from the research.
How often do IRB/IEC reviews take place?
Before a site is allowed to start enrolling patients in a clinical trial, the IRB/IEC must review all study-related materials in an initial review. The IRB/IEC also performs periodic reviews—called continuing reviews—throughout the trial’s duration. Continuing reviews may take place at least once a year and include the entire trial, not just changes.
What is an IRB?
The International Council on Harmonisation (ICH) defines an institutional review board (IRB) as a group formally designated to protect the rights, safety and well-being of humans involved in a clinical trial by reviewing all aspects of the trial and approving its startup. IRBs can also be called independent ethics committees (IECs).
Who is responsible for ensuring compliance with IRB/IEC procedures?
The principal investigator (PI) is responsible for ensuring compliance with any IRB/IEC procedures or requirements. He or she may deviate from the study protocol without prior IRB/IEC approval only to eliminate an immediate safety hazard to a study participant. In addition, the PI must notify the IRB/IEC of any departures from the protocol as soon as possible.
How many members should an IRB have?
Per the FDA, an IRB/IEC should have: At least five members. Members with varying backgrounds. At least one member who represents a non-scientific area (a lay member).
Why should IRB/IEC review payment?
3.1.8 The IRB/IEC should review both the amount and method of payment to subjects to assure that neither presents problems of coercion or undue influence on the trial subjects. Payments to a subject should be prorated and not wholly contingent on completion of the trial by the subject.
What are the responsibilities of an IRB?
Responsibilities. 3.1.1 An IRB/IEC should safeguard the rights, safety, and well-being of all trial subjects. Special attention should be paid to trials that may include vulnerable subjects. trial protocol (s)/amendment (s), written informed consent form (s) and consent form updates that the investigator proposes for use in the trial, ...
How many members should be in an IRB?
It is recommended that the IRB/IEC should include: (a) At least five members. (b) At least one member whose primary area of interest is in a nonscientific area. (c) At least one member who is independent of the institution/trial site. Only those IRB/IEC members who are independent of the investigator and the sponsor of the trial should vote/provide ...
How often should IRB review a trial?
3.1.4 The IRB/IEC should conduct continuing review of each ongoing trial at intervals appropriate to the degree of risk to human subjects, but at least once per year.
Who can ask the IRB to provide its written procedures and membership lists?
The IRB/IEC may be asked by investigators, sponsors or regulatory authorities to provide its written procedures and membership lists.
Who should vote on IRB review?
3.2.4 Only members who participate in the IRB/IEC review and discussion should vote/provide their opinion and/or advise.
How long should IRB keep records?
The IRB/IEC should retain all relevant records (e.g., written procedures, membership lists, lists of occupations/affiliations of members, submitted documents, minutes of meetings, and correspondence) for a period of at least 3 years after completion of the trial and make them available upon request from the regulatory authority (ies).
