Things to Remember
- A chlorate is an anionic group with one central Chlorine atom and three oxygen atoms.
- Chlorates can also refer to compounds containing chlorate ions.
- The oxidation state of the chlorate ion is +5. ...
- It is a very strong oxidizing agent, used in the paper industry to bleach paper pulp.
- Chlorates are prepared in the laboratory by adding chlorine to metal hydroxide. ...
...
Chlorate.
| Names | |
|---|---|
| Related compounds | chloride hypochlorite chlorite perchlorate |
What are some examples of chlorates?
Examples of chlorates include potassium chlorate, KClO 3 sodium chlorate, NaClO 3 magnesium chlorate, Mg(ClO 3) 2
What is chlorate ion and how is it formed?
Chlorate ion is a known byproduct of the drinking water disinfection process, forming when sodium hypochlorite or chlorine dioxide are used in the disinfection process. A number of compounds can react to release chlorate ion in water, including some in herbicides, fireworks and other explosives.
What is the difference between chlorine and chlorate?
/ Chlorate. The term “chlorate” most commonly refers only to chlorine in the +5 oxidation state, or chlorate ion. Chlorate ion is a known byproduct of the drinking water disinfection process, forming when sodium hypochlorite or chlorine dioxide are used in the disinfection process.
What is the chemical formula for chlorate?
Chlorate or the chlorate ion has a molecular formula ClO3 –. Chlorates are the salts of chloric acid. Chlorate when accompanied by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. Metal chlorates can be prepared by adding chlorine to metal hydroxides such as the KOH.
See more
What is chlorate made of?
Chlorate is formed by the electrolysis of a potassium chloride solution, when conducted in such a manner that the alkali formed around the cathode comes into contact with the chlorine set free at the anode, the temperature being kept above 50° C.
What element is ClO3?
Chlorine trioxide | ClO3 - PubChem.
What is the formula for chlorate?
ClO3−Chlorate / FormulaThe chlorate anion has the formula ClO3−. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid.
What elements are in sodium chlorate?
The chemical formula for sodium chlorate is NaClO3. It is a white crystalline solid form of cubic crystals with hygroscopicity. It decomposes slowly at about 300oC to oxygen and salt. Sodium chlorate is produced electrolytically from sodium chloride.
How is clo3 formed?
Chlorate is produced during bleaching with chlorine dioxide and has environmental implications. It is formed from the reaction of hypochlorous acid with chlorite ions (Ni et al., 1993; Germgard et al., 1981; Asplund and Germgard, 1991; Lindgren and Nilsson, 1975; Bergnor et al., 1987).
Is clo3 a molecule?
0:041:39ClO3- Molecular Geometry / Shape and Bond Angles - Chlorate IonYouTubeStart of suggested clipEnd of suggested clipMinus let's find the molecular geometry or shape. Looking at the lewis structure. We can see thatMoreMinus let's find the molecular geometry or shape. Looking at the lewis structure. We can see that there are three oxygens. And there's one lone pair of electrons.
Is chlorine a chlorate?
Using this convention, "chlorate" means any chlorine oxyanion. Usually, "chlorate" refers only to chlorine in the +5 oxidation state.
Where is chlorate found?
Chlorate is an inorganic compound that is a known byproduct of the drinking water disinfection process, forming when sodium or calcium hypochlorite (chlorine) or chlorine dioxide are used in the disinfection process.
What is the use of chlorate?
Chlorate is used in explosives and also as a pesticide. Hypochlorite and chlorine dioxide use as disinfectants are by far the principal sources in drinking water.
How do you name chlorate?
Chlorate or the chlorate ion has a molecular formula ClO3–. Chlorates are the salts of chloric acid. Chlorate when accompanied by a Roman numeral in parentheses, e.g. chlorate(VII), refers to a particular oxyanion of chlorine.
What is the difference between chlorate and chlorite?
Chlorates are used in the manufacture of dyes, matches, fireworks, disinfectants, and for tanning and finishing leather. Chlorite is a compound that contains the chlorine dioxide anion (ClO2-), with chlorine in the +3 oxidation state. Chlorites are salts of chlorous acid.
What is formula of sodium chlorate?
NaClO3Sodium chlorate / FormulaSodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride.
Is ClO3 ionic or covalent?
The compounds which are formed by cations and anions are known as ionic compounds. NaClO3 is formed by Na+ion and ClO3– ion where ClO3– ion is polyatomic ion or radical. ClO3– is formed by covalent bonds.
What is the oxidation number of Cl in ClO3 -?
+5In the chlorate ion (ClO3-), the oxidation number of Cl +5, and the oxidation number of O is -2. In a neutral atom or molecule, the sum of the oxidation numbers must be 0.
What is cio3 in chemistry?
Chlorate Formula Chlorate or the chlorate ion has a molecular formula ClO3–. Chlorates are the salts of chloric acid.
What is the charge of ClO3?
The formal charge of ClO3– is -1.
What does "chlorate" mean in chemistry?
If a Roman numeral in brackets follows the word "chlorate", this indicates the oxyanion contains chlorine in the indicated oxidation state, namely: Using this convention, "chlorate" means any chlorine oxyanion. Commonly, "chlorate" refers only to chlorine in the +5 oxidation state.
How to make metal chlorates?
Metal chlorates can be prepared by adding chlorine to hot metal hydroxides like KOH :
What is the formula for chlorate anion?
The chlorate anion has the formula ClO−. 3. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid.
Where is chlorate found?
A recent study has discovered the presence of natural chlorate deposits around the world, with relatively high concentrations found in arid and hyper-arid regions. The chlorate was also measured in rainfall samples with the amount of chlorate similar to perchlorate.
Is chlorate a Lewis structure?
Structure and bonding. The chlorate ion cannot be satisfactorily represented by just one Lewis structure, since all the Cl–O bonds are the same length (1.49 Å in potassium chlorate ), and the chlorine atom is hypervalent. Instead, it is often thought of as a hybrid of multiple resonance structures :
Can chlorate be used in pyrotechnics?
Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability.
Is perchlorate a part of the biogeochemistry cycle?
It is suspected that chlorate and perchlorate may share a common natural formation mechanism and could be a part of the chlorine biogeochemistry cycle. From a microbial standpoint, the presence of natural chlorate could also explain why there is a variety of microorganisms capable of reducing chlorate to chloride.
How is chlorate formed?
Chlorate is produced during bleaching with chlorine dioxide and has environmental implications. It is formed from the reaction of hypochlorous acid with chlorite ions ( Ni et al., 1993; Germgard et al., 1981; Asplund and Germgard, 1991; Lindgren and Nilsson, 1975; Bergnor et al., 1987 ). Chlorate does not react with the pulp and also represents a loss of bleaching potential ( Nilsson, 1974; Rapson and Anderson, 1977; Reeve and Rapson, 1981; Germgrad et al., 1981; Bergnor et al., 1987 ). Chlorate formation is proportional to chlorine dioxide consumption and is strongly dependent on the bleaching pH. The yield of chlorate can be as much as 50 mol% of the chlorine dioxide used ( Germgard et al., 1981 ):
What is the average level of chlorate?
In the US EPA's ICR, which represents the most extensive data for chlorate, the median level of chlorate was 0.12 mg l −1 at plants using chlorine dioxide for disinfection.
What is sodium perchlorate?
Sodium perchlorate is manufactured electrolytically from sodium chlorate, which is manufactured from brine in the electrolysis process. Manufacture of sodium chlorate is a potential source of perchlorate because perchlorate is generated as an unintended by-product in the electrolysis process. Sodium chlorate is used as herbicide, mainly as a defoliant. It was reported that perchlorate content in one chlorate defoliant was 24 mg kg −1. It was also reported that perchlorate is generated as an unintended by-product in electrolysis processes other than those for the manufacture of perchlorate and chlorate.
What is the purpose of hypochlorite solution?
Hypochlorite solution, which is used as a disinfectant in water purification processes, is known to contain perchlorate alongside other oxihalides (e.g., chlorite, chlorate, and bromate) as impurities. Hypochlorite solutions used in water systems (PWSs or private water systems) are typically commercial sodium hypochlorite solutions or on-site hypochlorite solutions generated by the electrolysis of brine. It was reported that perchlorate concentrations in 32 commercial hypochlorite solutions and 6 hypochlorite solutions generated on-site that were used in water systems were in the range of 170–33 000 μg l −1 (median concentration, 2300 μg l −1) and 13–660 μg l −1 (median concentration, 32 μg l −1 ), respectively ( Table 3 ). Perchlorate concentrations in the hypochlorite solutions were higher in cases in which the levels of free available chlorines (FACs) in the solutions measured in the laboratory (8.0–16.4%) were lower. Perchlorate concentrations in the commercial hypochlorite solutions are considered to increase in accordance with the decay of FACs in the solutions. Another study showed that perchlorate concentrations in hypochlorite solutions increase during storage. In general, the concentration of perchlorate originating from hypochlorite solution is low in finished drinking water (0.2–0.4 μg l −1 ). However, in two cases in Japan, the perchlorate concentration in finished drinking water in a PWS was found to be ≥1 μg l −1 (6.1 μg l −1 in October 2006 and 1.1 μg l −1 in February 2007). The hypochlorite disinfectant was the suspected source in both cases as perchlorate concentrations in the raw waters were low (<0.05 μg l −1) and those in the hypochlorite solutions were high (13 000 μg l −1 in February 2007). Such cases highlight the risks of perchlorate contamination in water systems by hypochlorite solutions with low FACs and perchlorate accumulation.
What are the compounds of indium phosphate?
Indium (III) compounds of polyoxyanions such as nitrate, sulfate, phosphate, and chlorate are well known.1,4 Some of the work on indium phosphates in the late 1990s centered on developing different three-dimensional structures using organic templates. 233–242 Indium phosphonate derivatives have also been investigated as building blocks for preparing materials with well-defined internal spaces. 243–246
Is chlorate toxic to algae?
Chlorate is toxic to brown algae in marine waters ( Rosemarin et al., 1990 ). It was responsible for the destruction of a large area of bladder wrack in the Baltic Sea ( Lehtinen et al., 1988 ). Malmquist et al. (1991) have reported that it is easily removed during biological treatment of mill effluent if an anaerobic stage is included in the treatment.
How to determine halogens?
The halogens may be determined polarographically by reduction of their halate form (except for chlorate). Bromide, for example, may be determined by DPP after oxidation to the bromate species yielding a cathodic current at ∼−1.53 V versus SCE. The detection limit is ∼1×10 −6 mol l −1. Possible interferences would arise from iodate reduction.
What is chlorate in water?
What is chlorate? The term “chlorate” most commonly refers only to chlorine in the +5 oxidation state, or chlorate ion. Chlorate ion is a known byproduct of the drinking water disinfection process, forming when sodium hypochlorite or chlorine dioxide are used in the disinfection process.
How much chlorate ion is harmful to the thyroid?
Environmental Protection Agency’s current reference concentration indicates that ongoing exposure to chlorate ion at levels of more than 210 parts per billion per day can lead to an enlarged thyroid.
How can water providers mitigate the production of chlorate ion during disinfection?
Water providers can mitigate the production of chlorate ion during disinfection by observing best practices such as avoiding exposure to light sources, minimizing storage time, maintaining proper pH and properly handling chemicals involved in the process.
What is the formula for chlorate?
Chlorate or the chlorate ion has a molecular formula ClO3 –. Chlorates are the salts of chloric acid. Chlorate when accompanied by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. Metal chlorates can be prepared by adding chlorine to metal hydroxides such as the KOH.
Is chlorate ion a Lewis structure?
Chlorate Ion Lewis Structure. Just one Lewis structure cannot satisfactorily represent the chlorate ion since all the Cl–O bonds are of the same length and the chlorine atom is hypervalent. Rather, it is often considered as a hybrid of multiple resonance structures as follows:
What is the chemical reaction of sodium chlorate?
SODIUM CHLORATE decomposes upon heating forming O2; reacts with strong acids forming toxic and explosive ClO2; reacts with many substances [Handling Chemicals Safely 1980 p. 833]; metal chlorates are oxidants in the presence of strong acid; liberates explosive chlorine dioxide gas; heating a moist metal chlorate and a dibasic organic acid liberates chlorine dioxide and carbon dioxide; mixtures of perchlorates with sulfur or phosphorus are explosives [Bretherick 1979 p. 100]; mixtures of the chlorate with ammonium salts ( ammonium thiosulfate ), powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806]. A combination of finely divided aluminum with finely divided bromates (also chlorates and iodates) of barium, calcium, magnesium, potassium, sodium or zinc can explode by heat, percussion, and friction [Mellor 2:310 1946-47]. Sodium chlorate and arsenic trioxide form a spontaneously flammable mixture [Ellern 1968 p. 51]. Mixtures of organic material and more than 10% sodium chlorate are sufficiently combustible to be hazardous at low relative humidity. Mixtures of organic material such as charcoal, sugar, flour, or shellac and sodium chlorate may be ignited by friction or shock [Chem. Safety Data Sheet SD-42 1951].
What is chlorate salt used for?
At one time, the chlorate salts, sodium chlorate and potassium chlorate, were used as medicinal agents to treat inflammatory and ulcerative lesions of the oral cavity and could be found in various mouthwash, toothpaste, and gargle preparations. /Former use/
What is a mixture of sodium chlorate and nitrobenzene?
A mixture /of sodium chlorate and nitrobenzene / is powerfully explosive. ... Mixtures with fibrous or absorbent organic materials ( charcoal, flour, shellac, sawdust, sugar) are hazardous and can be caused to explode by static friction or shock. ...
How many people were exposed to sodium chlorate in 1981?
NIOSH (NOES Survey 1981-1983) has statistically estimated that 28,583 workers (5,207 of these were female) were potentially exposed to sodium chlorate in the US (1). The NOES Survey does not include farm workers.
What is the code for sodium chlorate?
For Sodium chlorate (USEPA/OPP Pesticide Code: 073301) ACTIVE products with label matches. /SRP: Registered for use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
What is a mixture of combustible materials?
Mixtures with combustible materials are very flammable and may be ignited by friction. It is used for making herbicides, explosives, dyes, matches, inks, cosmetics, pharmaceuticals, defoliants, paper, and leather. Sodium chlorate is an inorganic sodium salt that has chlorate as the counter-ion.
Is sodium chlorate a defoliant?
Sodium chlorate is exempted from the requirement of a tolerance for residues in or on the following raw agricultural commodities when used as a defoliant, desiccant, or fungicide in accordance with good agricultural practice: beans, dry, edible; corn, fodder; corn, forage; corn, grain; cottonseed; flaxseed; flax, straw; guar beans; peas, southern; peppers, chili; potatoes; rice; rice, straw; safflower, grain; sorghum, grain; sorghum, fodder; sorghum, forage; soybeans; and sunflower seed.
Is Cameo Chemicals copyrighted?
CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.
Is magnesium chlorate soluble in water?
Magnesium chlorate appears as white deliquescent crystals or powder. Soluble in water and denser than water. Poses a dangerous fire risk when in contact with organic materials or heat. May be irritating to skin, eyes and mucous membranes. Used to make other chemicals.
Overview
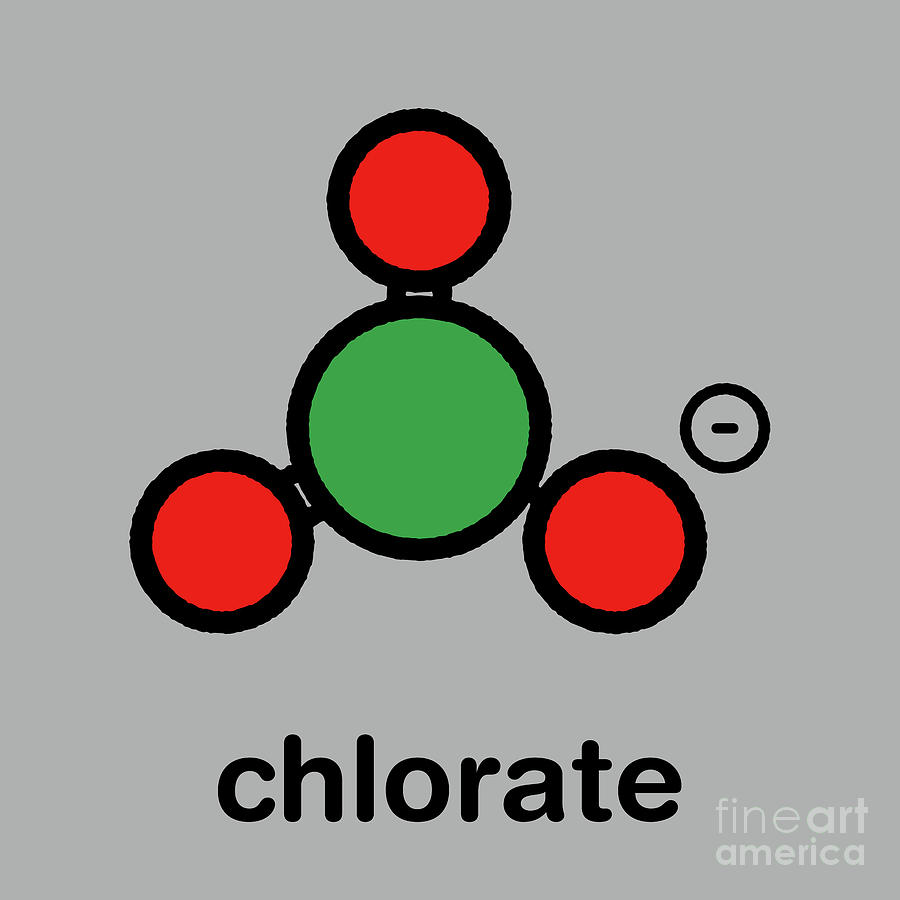
The chlorate anion has the formula ClO 3. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine.
Structure and bonding
The chlorate ion cannot be satisfactorily represented by just one Lewis structure, since all the Cl–O bonds are the same length (1.49 Å in potassium chlorate ), and the chlorine atom is hypervalent. Instead, it is often thought of as a hybrid of multiple resonance structures:
Preparation
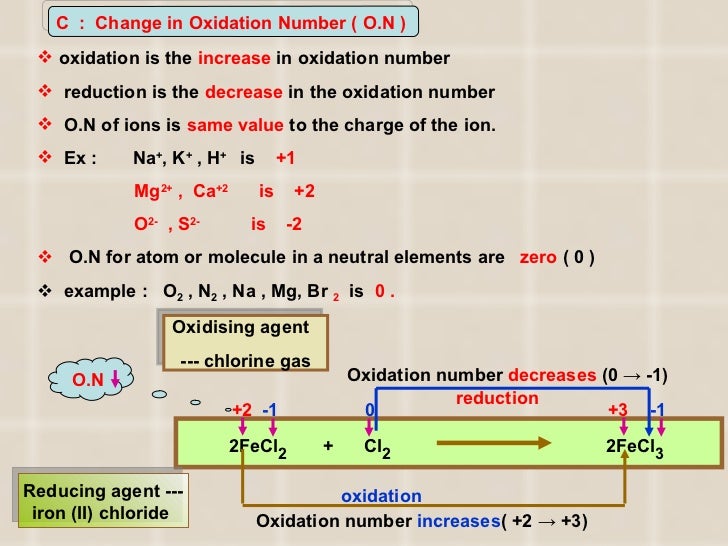
Metal chlorates can be prepared by adding chlorine to hot metal hydroxides like KOH:
3 Cl2 + 6 KOH → 5 KCl + KClO3 + 3 H2O
In this reaction, chlorine undergoes disproportionation, both reduction and oxidation. Chlorine, oxidation number 0, forms chloride Cl (oxidation number −1) and chlorate(V) ClO 3 (oxidation number +5). The reaction of cold aqueous metal hydroxides with chlorine produces the chloride …
Natural occurrence
A recent study has discovered the presence of natural chlorate deposits around the world, with relatively high concentrations found in arid and hyper-arid regions. The chlorate was also measured in rainfall samples with the amount of chlorate similar to perchlorate. It is suspected that chlorate and perchlorate may share a common natural formation mechanism and could be a part of the chlorine biogeochemistry cycle. From a microbial standpoint, the presence of natural …
Compounds (salts)
Examples of chlorates include
• potassium chlorate, KClO3
• sodium chlorate, NaClO3
• magnesium chlorate, Mg(ClO3)2
Toxicity
Chlorates are relatively toxic, though they form generally harmless chlorides on reduction.
External links
• "Chlorates" . Encyclopædia Britannica. Vol. 6 (11th ed.). 1911. p. 254.