
What exactly is an atom?
What is an atom? An atom is the basic building block of chemistry. It is the smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element.
What is atoms in simple words?
An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Is an atom matter or energy?
Just like bricks are the building blocks of a home, atoms are the building blocks of matter. Matter is anything that has mass and takes up space (volume). All matter is made up of atoms. The atom has a nucleus, which contains particles of positive charge (protons) and particles of neutral charge (neutrons).
Are humans made of atoms?
About 99 percent of your body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. You also contain much smaller amounts of the other elements that are essential for life.
How many atoms are on the earth?
133,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000According to the US Department of Energy's Jefferson Lab, the answer is: 133,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000. That answer comes from an estimation of the number of atoms in each of Earth's elements, like Iron, Oxygen, Silicon, Magnesium, Sulfur … etc.
Can atoms be destroyed?
The mass of an atom can neither be created nor destroyed.
Are atoms alive?
Atoms are not living things; they do not need food, water, and air; and they do not reproduce themselves. Cells are alive. Cells are bigger than atoms. We can see cells with a microscope.
How long does an atom last?
Ultimately, even these stable atoms have a limit imposed by the lifetime of proton (>1025 years). Remember, though, that the best estimate of the present age of the universe is the much smaller number of 1010 years, so for all practical purposes, atoms are forever.
What is an atom?
An atom is the basic building block of chemistry. It is the smallest unit into which matter can be divided without the release of electrically char...
Are all atoms the same size?
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would me...
What does the mass of an atom consist of?
The mass of an atom consists of the mass of the nucleus plus that of the electrons. That means the atomic mass unit is not exactly the same as the...
How is the atomic number of an atom defined?
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z), which is defined as the number of units...
What is the difference between a neutral and a neutral atom?
For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance.
What is matter made of?
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
How many protons are in a nucleus?
The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive.
Why do we use complementary pictures of the atom?
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
How much space does the nucleus take up?
It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10 −15 metre.
How many atoms are in a row?
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. The radius of an atom measures 1–2 Å.
Which part of an atom contains the most mass?
The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms.
What are the components of an atom?
Components. Electrons and a compact nucleus of protons and neutrons. An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across.
What is the nucleus made of?
The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge.
How are electrons attracted to protons?
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another.
What is an atom that has more or fewer electrons called?
If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions .
How much oxygen is in tin oxide?
For example, there are two types of tin oxide: one is a black powder that is 88.1% tin and 11.9% oxygen, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the black oxide there is about 13.5 g of oxygen for every 100 g of tin, and in the white oxide there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. In these oxides, for every tin atom there are one or two oxygen atoms respectively ( SnO and SnO 2 ).
How small are atoms?
Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics —as if they were tennis balls, for example—is not possible due to quantum effects . Every atom is composed of a nucleus and one or more electrons bound to the nucleus.
Why did Rutherford and Marsden have doubts about the Thomson model?
Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles ( these are positively-charged particles emitted by certain radioactive substances such as radium ). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn't think he'd run into this same problem because alpha particles are much heavier than electrons. According to Thomson's model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle, and the electrons are so lightweight they should be pushed aside effortlessly by the much heavier alpha particles. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully.
How are electrons attracted to the nucleus?
Electrons are attracted to the nucleus by protons that reside there… one electron per proton. Protons have a single unit of positive charge equal, but opposite to, the charge on electrons. Like electrons, protons can be modeled as particles or fields… well, the proton's matter field breaks down into the strong field and the lepton field, which produces quarks and gluons. Protons have 1836 times the rest mass of an electron. It is the number of protons that determines which element the atom is.
What is the smallest unit of an element?
Atoms are the smallest units of elements. Each atom of an element has the properties of that element.
How are electrons modeled?
Electrons can also be modeled as a field, by mathematical equations which simulate the electrons properties. With the field view, electrons are “clouds" of probabilities “smeared” out over space. By doing a particular math operation, fields can be resolved into a single “quanta" which is particle-like in nature, but is not truly considered a particle.
Where are electrons concentrated?
Most of the mass as well as all the positive electric charge of an atom are concentrated in the nucleus . Electrons can be regarded as point-like charges having a mass of 9.10938291×10−31 kg and a negative electric charge of 1.602176565×10−19 coulomb .
What is the smallest particle of a substance that can take part in a chemical reaction?
An atom is the smallest indivisible particle of a substance that can take part in a chemical reaction.
Why does the Greek water thief only work?
The Greek “water thief” only works because molecules of water “can’t be divided that easily”. But atoms CAN be divided. The essential difference goes back to my first definition. Outside of an atom, it “makes sense” to talk about the “time and space” of something.
What do you see when you split a diamond?
The sunlight you do see when you look at the sun is the sun. If you split a diamond, the “edge” is a row of carbon atoms. Notice that a perfect diamond has “invisible” carbon atoms, not only at the edge but also inside.
What are the parts of an atom?
Most atoms have three different subatomic particles inside them: protons , neutrons , and electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. When people draw pictures of atoms, they show the electrons like satellites spinning round the Earth in orbits. In fact, electrons move so quickly that we never know exactly where they are from one moment to the next. Imagine them as super-fast racing cars moving so incredibly quickly that they turn into blurry clouds—they almost seem to be everywhere at once. That's why you'll see some books drawing electrons inside fuzzy areas called orbitals .
How do atoms make molecules and compounds?
So hydrogen atoms don't exist by themselves: instead, they pair up to make what is called a molecule of hydrogen. A molecule is the smallest amount of a compound: a substance made from two or more atoms.
What are isotopes?
The ordinary carbon we find in the world around us is sometimes called carbon-12. It has six protons, six electrons, and six neutrons, so its atomic number is 6 and its relative atomic mass is 12. But there's also another form of carbon called carbon-14, with six protons, six electrons, and eight neutrons. It still has an atomic number of six, but its relative atomic mass is 14. Carbon-14 is more unstable than carbon-12, so it's radioactive : it naturally disintegrates, giving off subatomic particles in the process, to turn itself into nitrogen. Carbon-12 and carbon-14 are called isotopes of carbon. An isotope is simply an atom with a different number of neutrons that we'd normally expect to find.
How do atoms make ions?
Atoms aren't just packets of matter: they contain electrical energy too. Each proton in the nucleus of an atom has a tiny positive charge ( electricity that stays in one place). We say it has a charge of +1 to make everything simple (in reality, a proton's charge is a long and complex number: +0.00000000000000000016021892 C, to be exact!). Neutrons have no charge at all. That means the nucleus of an atom is effectively a big clump of positive charge. An electron is tiny compared to a proton, but it has exactly the same amount of charge. In fact, electrons have an opposite charge to protons (a charge of −1 or −0.00000000000000000016021892 C, to be absolutely exact). So protons and electrons are a bit like the two different ends of a battery: they have equal and opposite electric charges. Since an atom contains equal number of protons and electrons, it has no overall charge: the positive charges on all the protons are exactly balanced by the negative charges on all the electrons. But sometimes an atom can gain or lose an electron to become what's called an ion. If it gains an electron, it has slightly too much negative charge and we call it a negative ion; it it loses an electron, it becomes a positive ion.
How many atoms are there in something?
If atoms are so tiny, there must be zillions and zillions of them in all the things around us... but how many are there, exactly?
How do we know atoms exist?
Artwork: Molecules are built from atoms: In the early 19th century, English chemist John Dalton (1766–1844) realized that atoms join together in simple ratios. Water forms when two hydrogens snap onto one oxygen. Chemical reactions like this make sense if the elements exist as simple building blocks: atoms, in other words.
How many protons are in an iron atom?
Cut apart a single atom of iron and you will find 26 protons and 30 neutrons clumped together in the nucleus and 26 electrons whizzing around the outside. An atom of gold is bigger and heavier. Split it open and you'll find 79 protons and 118 neutrons in the nucleus and 79 electrons spinning round the edge.
What are the properties of protons and neutrons?
One of those properties is mass, or how much stuff they contain. Protons and neutrons have masses of 1 atomic mass unit (AMU). And electrons have a tiny, tiny mass of 1/1836th of the protons and neutrons.
Why are electrons considered fundamental particles?
Electrons are fundamental particles, because they can't be broken down any more. But protons and neutrons actually contain smaller particles inside them called quarks. You don't need to know much about them, unless you're a physics major in college. But just knowing they exist means you understand atoms better than most people.
Why do we know that atoms are there?
We know atoms are there because we can see their effects . We can see the results of chemical reactions and figure out how they work. We can also use a mass spectrometer to weigh atoms, and in modern times, we can create images of atoms using electron microscopes, which fire fast electrons at an atom, causing them to bounce off in different directions in which the way they bounce off allows an image to be created.
How many atomic mass units does a proton have?
Protons have a mass of 1 atomic mass unit, and a charge of +1. Electrons have a mass of 1/1836th atomic mass units and a charge of -1. And neutrons have a mass of 1 atomic mass unit and a charge of 0; they're neutral. We know atoms are there because we can see their effects.
How do we take pictures of atoms?
But these days, we can even take photos of atoms using some incredible technology called an electron microscope. An electron microscope fires fast electrons at an atom, causing them to bounce off in different directions in which the way they bounce off allows an image to be created—kind of like how dolphins use sonar to take pictures of the sea floor their brains can understand.
Why do chemical reactions happen?
When chemical reactions happen, it's because atoms are rearranging the way they're connected to other atoms. And by understanding what atoms are made of, we can explain why those chemical reactions happen. For example, maybe one atom gives an electron to another atom.
Where are protons and neutrons located in an atom?
The protons, and neutrons are found in the very center of the atom , which is called the nucleus. And the electrons orbit around the outside. Atoms are mostly empty space, but you can't put your hand through solid objects because the intermolecular forces, or forces between molecules, are too strong.
How much more massive is a proton than an electron?
However, one proton is about 1,835 times more massive than an electron. Atoms always have an equal number of protons and electrons, and the number of protons and neutrons is usually the same as well. Adding a proton to an atom makes a new element, while adding a neutron makes an isotope, or heavier version, of that atom.
How many protons are in an atom?
The number of protons in an atom is unique to each element. For example, carbon atoms have six protons, hydrogen atoms have one and oxygen atoms have eight . The number of protons in an atom is referred to as the atomic number of that element. The number of protons also determines the chemical behavior of the element. Elements are arranged in the Periodic Table of the Elements in order of increasing atomic number.
What are the three particles that make up an atom?
We now know that atoms are made up of three particles: protons, neutrons and electrons — which are composed of even smaller particles, such as quarks.
What are the building blocks of matter?
Reference Article: Facts about atoms, the building blocks of matter. Atoms consist of a nucleus made of protons and neutrons orbited by electrons. (Image credit: Shutterstock) Atoms are the basic units of matter and the defining structure of elements. The term "atom" comes from the Greek word for indivisible, because it was once thought ...
What is the electron cloud model?
The inner orbitals surrounding the atom are spherical but the outer orbitals are much more complicated. An atom's electron configuration refers to the locations of the electrons in a typical atom.
Where do protons and neutrons reside?
Protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus. The electron cloud has a radius 10,000 times greater than the nucleus, according to the Los Alamos National Laboratory.
How did Thomson discover the electron?
He was able to determine the existence of electrons by studying the properties of electric discharge in cathode-ray tubes. According to Thomson's 1897 paper, the rays were deflected within the tube, which proved that there was something that was negatively charged within the vacuum tube. In 1899, Thomson published a description of his version of the atom, commonly known as the "plum pudding model." An excerpt of this paper is found on the Chem Team site. Thomson's model of the atom included a large number of electrons suspended in something that produced a positive charge giving the atom an overall neutral charge. His model resembled plum pudding, a popular British dessert that had raisins suspended in a round cake-like ball.
How do we know if atoms exist?
How do we know they exist? Through chemical reactions we can witness their effects. Through mathematical equations combined with indirect observations, we can calculate their various sizes. And finally, with the aid of new technologies such as the Scanning Tunnelling Microscope, atoms can now be seen.
What are the building blocks of chemistry?
Atoms are the fundamental building blocks of chemistry. Just like baked goods are made of a collection of different types of ingredients, matter itself, is made of a collection of different types of atoms.
Why are atoms useful?
The idea of atoms helped them make predictions and perform cleaner chemical reactions.
How can salt be extracted from seawater?
Let’s fast forward several hundred years and hop over to the Arabic world. You probably know that salt can be extracted from seawater by simply boiling it dry. People have been doing this forever but Alchemist Jabir ibn Hayyan and those that followed his work, took the science extraction to a whole new level.
What did Democritus call the particles that were indestructible?
Instead he insisted that at some point you would reach particles so small and so indestructible they could not be divided any further. He called them “Atomos” or atoms, which means “uncuttable”.
What elements react to form a brown powder?
The elements oxygen and iron can react to form a brown powder. Oxygen and mercury react to form a toxic orange powder. Oxygen and hydrogen react to form a clear refreshing liquid, and so on.
What did the scientists discover about the process of filtration?
They found that crude starting materials could be divided into multiple incredibly pure substances. Pure meaning they appeared consistent all the way through unlike the complex mixtures of matter we often find in nature.
Overview
Properties
By definition, any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons (hydrogen-1, by far the most common form, also called protium), one neutron (deuterium), two neutrons (tritium) and more than two neutrons. Th…
History of atomic theory
The basic idea that matter is made up of tiny, indivisible particles appears in many ancient cultures such as those of Greece and India. The word atom is derived from the ancient Greek word atomos (a combination of the negative term "a-" and "τομή," the term for "cut") that means "uncuttable". This ancient idea was based in philosophical reasoning rather than scientific reasoning; modern atomic theory is not based on these old concepts. Nonetheless, the term "ato…
Structure
Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are the electron, the proton and the neutron.
The electron is by far the least massive of these particles at 9.11×10 kg, with a negative electrical charge and a size that is too small to be measured using available techniques. It was the lightes…
Identification
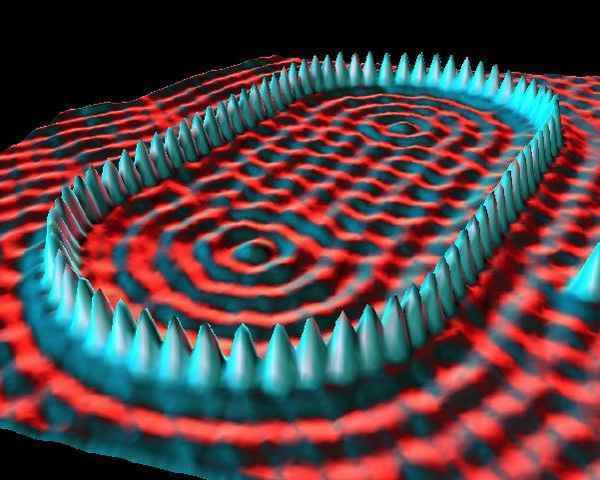
While atoms are too small to be seen, devices such as the scanning tunneling microscope (STM) enable their visualization at the surfaces of solids. The microscope uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two biased electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode i…
Origin and current state
Baryonic matter forms about 4% of the total energy density of the observable universe, with an average density of about 0.25 particles/m (mostly protons and electrons). Within a galaxy such as the Milky Way, particles have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 10 to 10 atoms/m . The Sun is believed to be inside the Local Bubble, so the density in the solar neighborhood is only about 10 atoms/m . Stars form fro…
See also
• History of quantum mechanics
• Infinite divisibility
• List of basic chemistry topics
• Motion
• Timeline of atomic and subatomic physics
Bibliography
• Oliver Manuel (2001). Origin of Elements in the Solar System: Implications of Post-1957 Observations. Springer. ISBN 978-0-306-46562-8. OCLC 228374906.
• Andrew G. van Melsen (2004) [1952]. From Atomos to Atom: The History of the Concept Atom. Translated by Henry J. Koren. Dover Publications. ISBN 0-486-49584-1.