
What happens when carbonate reacts with acid?
When carbonate reacts with acid , it forms corresponding salt, CO2 & H2O. Sodium carbonate ( Metal carbonate) reacts with hydrochloric acid, it forms sodium chloride salt , carbon dioxide & water. Metal carbonates react with acid to form metal salt, carbon dioxide and water.
What happens when a metal carbonate is added to Salt?
The salt consists of the metal cation and the anionic form of the acid (e.g. chloride ion from HCl) The metal carbonate dissolves. If the salt formed is water-soluble, the colourless solution turns to the colour of the metal ion (aq). Otherwise, a precipitate having the colour of the metal ion (s) is seen. Colourless gas bubbles are evolved.
What happens when Na2CO3 reacts with HCl?
When carbonate reacts with acid , it forms corresponding salt, CO2 & H2O. Na2CO3 (s) + 2HCl ——→ 2NaCl (sq) + H20(l) + CO2 (g) Sodium carbonate ( Metal carbonate) reacts with hydrochloric acid, it forms sodium chloride salt , carbon dioxide & water.
What happens when carbon dioxide is gassed from a metal?
When carbon dioxide off gassed, only the created water remained along with new metal salt. Take an example - when sodium bicarbonate react with sulphuric acid it forms sodium sulphate , water and releases carbon dioxide gas . Is your state's climate "normal?"
What happens when an acid reacts with a carbonate and or bicarbonate?
Why do acids react with carbonates?
What can displace hydrogen ions from acids?
Which atoms are attracted to the carbonate ion?
What type of reaction is hydrogen in?
What is salt made of?
Which ion can attack one of the other negatively charged oxygen atoms to form a second H-O?
See 4 more
About this website
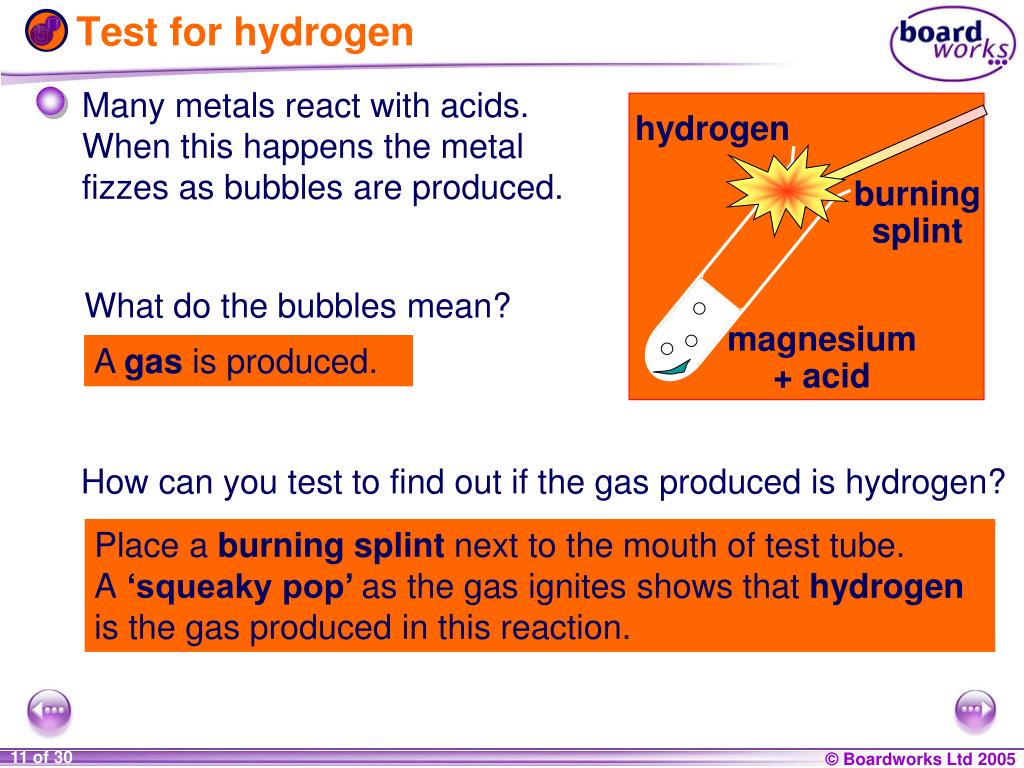
Write Balanced Equation for a Metal that Evolves a Gas Which Burns with ...
Write Balanced Equation for a Metal that Evolves a Gas Which Burns with a Pop Sound When Boiled with Alkali Solutions. - Chemistry
Select the correct answer from the list given in brackets: - Toppr Ask
Click here👆to get an answer to your question ️ Select the correct answer from the list given in brackets:When dilute sodium chloride is electrolysed using graphite electrodes, the cation is discharged at the cathode most readily. [Na^+, OH^-, H^+, Cl^-]
The electrolysis of acidified water is an example of - Toppr Ask
Click here👆to get an answer to your question ️ The electrolysis of acidified water is an example of .
Arrange the following according to the instructions given in brackets ...
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students.
Among the fallowing compounds identify, the compound that has all three ...
Among the fallowing compounds identify, the compound that has all three bonds (ionic, covalent and ... (C) Sodium hydroxide (D) Calcium chloride
Arrange the following as per the instruction given in the brackets: Na ...
Arrange the following as per the instruction given in the brackets: Na, K, Li (Increasing atomic size)
What happens when an acid reacts with a carbonate and or bicarbonate?
When an Acid reacts with a carbonate and or bicarbonate, caborn dioxide (CO2) is liberated and when it reacts with a metal (active metal) Hydrogen gas (H2) is liberated.
Why do acids react with carbonates?
Acids react with carbonates because acids produce hydrogen ions.
What can displace hydrogen ions from acids?
Active metals can displace hydrogen ions from acids, while inactive metals cannot. The salt produced when an acid reacts with a metal differs, in that the type of salt produced depends on the type of acid used for the reaction. Sulfuric acid produces sulfate salts, while hydrochloric acid produces chloride salts.
Which atoms are attracted to the carbonate ion?
The hydrogen ion is therefore attracted to the carbonate ion and first reacts with one of the negatively charged oxygen atoms which make up the carbonate ion, to form an H—O bond. This produces the hydrogen carbonate (bicarbonate) ion;-
What type of reaction is hydrogen in?
This type of chemical reaction is known as a single displacement reaction, where an element displaces another in a compound. The hydrogen in acids is displaced by the metals to produce hydrogen gas.
What is salt made of?
The salt consists of the metal cation and the anionic form of the acid (e.g. chloride ion from HCl)
Which ion can attack one of the other negatively charged oxygen atoms to form a second H-O?
Since the bicarbonate ion is still electron-rich, another hydrogen ion can likewise attack one of the other negatively charged oxygen atoms to form a second H—O bond which produces carbonic acid;-
What gas does sodium bicarbonate react with?
Take an example - when sodium bicarbonate react with sulphuric acid it forms sodium sulphate , water and releases carbon dioxide gas .
What will react with water to form acids?
Non-metal oxides will react with water to form acids.
What reacts differently with dilute acid?
2. Metals, above and below Hydrogen in the electrochemical series , react differently with with dilute ,oxidising inorganic acid like HNO3. For a metal reactions are in different concentrations of acid.
What happens when a double displacement reaction occurs?
A double displacement reaction occurs from combination of ions in the aqueous solution. Therefore, Na displaces Mg to form sodium sulphate + ppt of magnesium carbonate, as—->
Do non metals react with dilute acids?
Non metals do not react with dilute acids.
Does Ni-Cr-Mo react with HCl?
Ni-Cr-Mo alloys such as Hastelloy C do not react with dilute HCl. If the acid concentration goes beyond dilute and/or the temperature becomes high, then the HCl may react with the steel.
Is baking soda an acid or base?
A standard acid-base reaction occurs, as sodium bicarbonate (aka baking soda) is a weak base. You’ll get an aqueous solution of the salt (sodium chloride in this case), water and carbon dioxide gas given off as bubbles out of the solution:
What happens when an acid reacts with a carbonate and or bicarbonate?
When an Acid reacts with a carbonate and or bicarbonate, caborn dioxide (CO2) is liberated and when it reacts with a metal (active metal) Hydrogen gas (H2) is liberated.
Why do acids react with carbonates?
Acids react with carbonates because acids produce hydrogen ions.
What can displace hydrogen ions from acids?
Active metals can displace hydrogen ions from acids, while inactive metals cannot. The salt produced when an acid reacts with a metal differs, in that the type of salt produced depends on the type of acid used for the reaction. Sulfuric acid produces sulfate salts, while hydrochloric acid produces chloride salts.
Which atoms are attracted to the carbonate ion?
The hydrogen ion is therefore attracted to the carbonate ion and first reacts with one of the negatively charged oxygen atoms which make up the carbonate ion, to form an H—O bond. This produces the hydrogen carbonate (bicarbonate) ion;-
What type of reaction is hydrogen in?
This type of chemical reaction is known as a single displacement reaction, where an element displaces another in a compound. The hydrogen in acids is displaced by the metals to produce hydrogen gas.
What is salt made of?
The salt consists of the metal cation and the anionic form of the acid (e.g. chloride ion from HCl)
Which ion can attack one of the other negatively charged oxygen atoms to form a second H-O?
Since the bicarbonate ion is still electron-rich, another hydrogen ion can likewise attack one of the other negatively charged oxygen atoms to form a second H—O bond which produces carbonic acid;-
