Blinded Procedure: A description of the procedure, meaning that the administrator may know who the suspect is, but by virtue of the procedure’s administration, the administrator is unable to inadvertently provide cues to the witness. For example, the use of a folder or envelope to conceal an array from the administrator, blinds the procedure.
Full Answer
What is a single blind study in psychology?
In psychology, a single-blind study is a type of experiment or clinical trial in which the experimenters are aware of which subjects are receiving the treatment or independent variable, but the participants of the study are not. Reasons to Conduct a Single-Blind Study.
What is an example of a double blind study?
For example, let's imagine that researchers are investigating the effects of a new drug. In a double-blind study, the researchers who interact with the participants would not know who was receiving the actual drug and who was receiving a placebo. Let’s take a closer look at what we mean by a double-blind study and how this type of procedure works.
What is a blind or blinded experiment?
A blind or blinded-experiment is an experiment in which information about the test is masked (kept) from the participant, to reduce or eliminate bias, until after a trial outcome is known.
Is it necessary to remove blinds after completion of a study?
Removing a blind upon completion of a study is never mandatory, but is typically performed as a courtesy to study participants. Unblinding that occurs after the conclusion of a study is not a source of bias, because data collection and analysis are both complete at this time.

What are blind procedures?
In research, a blind procedure may be employed deliberately to enhance experimental control: A single blind is a procedure in which participants are unaware of the experimental conditions under which they are operating; a double blind is a procedure in which both the participants and the experimenters interacting with ...
What is single blind procedure in psychology?
Listen to pronunciation. (SING-gul-blind STUH-dee) A type of clinical trial in which only the researcher doing the study knows which treatment or intervention the participant is receiving until the trial is over. A single-blind study makes results of the study less likely to be biased.
What does it mean if an experiment is blind?
A blind — or blinded — study is an experiment in which information about the test is masked (kept) from the participant, to reduce or eliminate bias, until after a trial outcome is known.
Why are blind procedures used?
Overall, blind procedures help to minimize biases and produce accurate results, which can be used to assess the efficacy of medication and other treatments.
What is a double-blind procedure in psychology?
Listen to pronunciation. (DUH-bul-blind STUH-dee) A type of clinical trial in which neither the participants nor the researcher knows which treatment or intervention participants are receiving until the clinical trial is over. This makes results of the study less likely to be biased.
What is the difference between a blind and double-blind experiment?
Blinding or masking In a single-blind study, patients do not know which study group they are in (for example whether they are taking the experimental drug or a placebo). In a double-blind study, neither the patients nor the researchers/doctors know which study group the patients are in.
What does blinding mean in experimental design?
Blinding, in research, refers to a practice where study participants are prevented from knowing certain information that may somehow influence them—thereby tainting the results.
What is blind design in research?
The double-blind design describes an experimental procedure in which neither the participant nor the experimenter are aware of which group (i.e., experimental or control) each participant belongs to.
Why are blind trials good?
A double-blind study is one in which neither the participants nor the experimenters know who is receiving a particular treatment. This procedure is utilized to prevent bias in research results. Double-blind studies are particularly useful for preventing bias due to demand characteristics or the placebo effect.
Who should be blinded in a clinical trial?
All of the different parties involved in a clinical trial are possible sources of bias and can be blinded to ensure trial objectivity, including: The patient being treated. The clinical staff administering the treatment. The physician assessing the treatment.
How do you conduct a blind study?
Give one group of people the actual pill and one group a placebo, but don't tell the participants which pill they are receiving. Explanation: In order for a study to be "blind," the participants can't know which group they are sorted into.
What is the benefit of a blind study?
Blind studies eliminate possible sources of human bias and influence from the participants and/or researchers. They encourage accurate reporting an...
What is an example of a blind study?
One example of a blind study is for testing new products. The participants are unaware if they are being give the new product or the existing produ...
What is a blind study in research?
In research, a blind study is when one or more groups of involved parties do not know the independent variable being tested or into which group the...
Why is a single-blind study used?
A single-blind study is used when the researcher is the expert and would be the best person to perform the experiment. It can also be used if the r...
What is a blinded experiment?
Experiment in which information about the test is masked to reduce bias. In a blind or blinded experiment, information which may influence the participants of the experiment is withheld (masked or blinded) until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, ...
Why is blinding important?
In some fields, such as medicine, it is considered essential. In clinical research, a trial that is not a blinded trial is called an open trial .
What is unblinding in pharmacology?
Unblinding is common in blinded experiments , particularly in pharmacological trials. In particular, trials on pain medication and antidepressants are poorly blinded. Unblinding that occurs before the conclusion of a study is a source of experimental error, as the bias that was eliminated by blinding is re-introduced.
Why is unblinding a common cause?
A common cause for unblinding is the presence of side effects (or effects) in the treatment group. In pharmacological trials, premature unblinding can be reduced with the use of an active placebo, which conceals treatment allocation by ensuring the presence of side effects in both groups.
When does unblinding occur?
Unblinding occurs in a blinded experiment when information becomes available to one from whom it has been masked. In clinical studies, unblinding may occur unintentionally when a patient deduces their treatment group. Unblinding that occurs before the conclusion of an experiment is a source of bias.
Why do symphony orchestras have blind tests?
Blind tests can also be used to compare the quality of musical instruments.
How many blinded trials are there for acupuncture?
While the possibility of blinded trials on acupuncture is controversial, a 2003 review of 47 randomized controlled trials found no fewer than four methods of blinding patients to acupuncture treatment: 1) superficial needling of true acupuncture points, 2) use of acupuncture points which are not indicated for the condition being treated, 3) insertion of needles outside of true acupuncture points, and 4) the use of placebo needles which are designed not to penetrate the skin. The authors concluded that there was "no clear association between type of sham intervention used and the results of the trials."
What is blind sample testing?
Definition - What does Blind Sample Testing mean? A blind sample testing is a drug screen procedure that involves the collection, submission, and confirmation of an artificial urine specimen to ensure laboratory facilities meet test result standards mandated by the Department of Transportation (DOT).
How many blind samples can a lab receive?
A laboratory can receive up to a maximum of fifty blind samples on a quarterly basis with the proportion of the cumulative amount corresponding to seventy-five percent negative (no drugs), fifteen percent positive (one to five DOT tested drugs), and ten percent adulterated.
Why do we do double blind studies?
Reasons to Use a Double-Blind Study 1 First, since the participants do not know which group they are in, their beliefs about the treatment are less likely to influence the outcome. 2 Second, since researchers are unaware of which subjects are receiving the real treatment, they are less likely to accidentally reveal subtle clues that might influence the outcome of the research. 1
Why is double blinding important?
A double-blind study can be a useful research tool in psychology and other scientific areas. By keeping both the experimenters and the participants blind, bias is less likely to influence the results of the experiment.
What is treatment in psychology?
In a psychology experiment, the treatment is the level of the independent variable that the experimenters are manipulating. This can be contrasted with a single-blind study in which the experimenters are aware of which participants are receiving the treatment while the participants remain unaware. 1 .
Why is randomized double blind placebo considered the gold standard?
2 One of the reasons for this is the fact that random assignment reduces the influence of confounding variables.
Can you double blind in a psychotherapy experiment?
Double-blind experiments are simply not possible in some scenarios. For example, in an experiment looking at which type of psychotherapy is the most effective, it would be impossible to keep participants in the dark about whether or not they actually received therapy.
Can you take a sugar pill in a double blind study?
The rest of the subjects will receive an inactive placebo. With a double-blind study, the participants and the experimenters have no idea who is receiving the real drug and who is receiving the sugar pill.
Origins of the Double-Blind Study
One of the earliest recorded examples of the double-blinded study was conducted in 1784, by Friedrich Wilhelm von Hoven, a government health official in Germany. He wanted to test whether homeopathic drugs, which were popular at the time, were a legitimate form of medicine.
Double-Blind Control
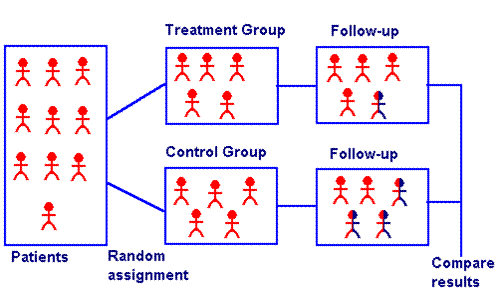
The control group plays an important role in experimental research. As previously mentioned, this group does not receive any special treatment during the course of a study. This group serves to represent how the population behaves in regular, everyday scenarios.
Randomization
Randomization plays a similarly important role in experimental research. Ultimately, researchers are trying to attribute any observed differences between the control and treatment groups to the conditions of the study. To do this, they need to be certain they can rule out other differences that might have influenced the results of the study.
The Process of Unblinding
Researchers frequently assess participants regarding the degree of their blindness. This is often done by asking participants questions about various aspects of the study that were withheld from them. If the participants exhibit no knowledge of this withheld information, then their blinding is considered perfect.
Premature Unblinding
Researchers aim to maximize blindness as much as possible. Premature blindness occurs when participants become privy to information that was withheld about the conditions of a study before it has ended.
Unblinding After the Study
Ideally, unblinding occurs after a study has ended. That way, biases that can arise from premature blinding are avoided. The unblinding of participants is often achieved by debriefing them to inform them of the nature of the study. This is particularly important if deception was used as a way of "blinding" them before the study begins.
What is single blind study?
In psychology, a single-blind study is a type of experiment or clinical trial in which the experimenters are aware of which subjects are receiving the treatment or independent variable, but the participants of the study are not.
Why is single blind study important?
A single-blind study can help prevent this or minimize the effects of such demand characteristics.
:max_bytes(150000):strip_icc()/165564292-56a0dcb65f9b58eba4b48bce.jpg)
Overview
Unblinding
Unblinding occurs in a blinded experiment when information becomes available to one from whom it has been masked. In clinical studies, unblinding may occur unintentionally when a patient deduces their treatment group. Unblinding that occurs before the conclusion of an experiment is a source of bias. Some degree of premature unblinding is common in blinded experiments. When a blind is imperfect, its success is judged on a spectrum with no blind (or complete failure of blindin…
History
The first blind experiment was conducted by the French Royal Commission on Animal Magnetism in 1784 to investigate the claims of mesmerism as proposed by Charles d'Eslon, a former associate of Franz Mesmer. In the investigations, the researchers (physically) blindfolded mesmerists and asked them to identify objects that the experimenters had previously filled with "vital fluid". The subjects were unable to do so.
Background
A number of biases are present when a study is insufficiently blinded. Patient-reported outcomes can be different if the patient is not blinded to their treatment. Likewise, failure to blind researchers results in observer bias. Unblinded data analysts may favor an analysis that supports their existing beliefs (confirmation bias). These biases are typically the result of subconscious influences, and are present even when study participants believe they are not influenced by them.
Applications
Blinding is considered essential in medicine, but is often difficult to achieve. For example, it is difficult to compare surgical and non-surgical interventions in blind trials. In some cases, sham surgery may be necessary for the blinding process. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constrains.
Studies of blinded pharmacological trials across widely varying domains find evidence of high le…
See also
• Allocation concealment
• Black boxing
• Jadad scale
• Metascience
• Royal Commission on Animal Magnetism