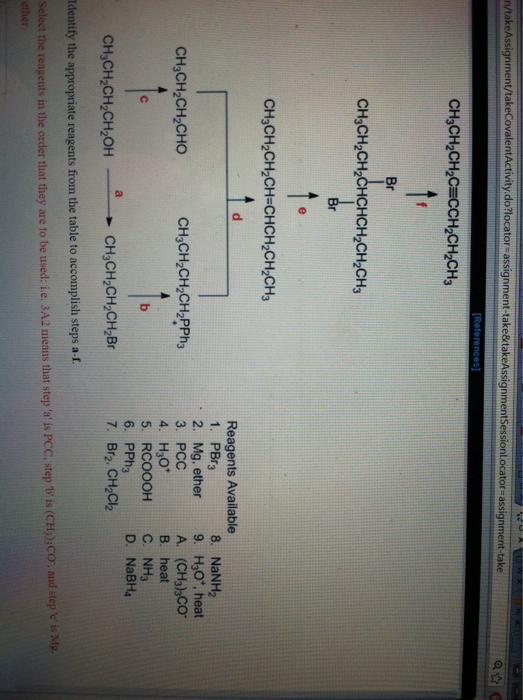
What is a reagent used for?
A reagent may be used to find out whether or not a specific chemical substance is present by causing a reaction to occur with it. Reagents may be compounds or mixtures. In organic chemistry, most are small organic molecules or inorganic compounds.
What does reagent grade mean in chemistry?
What Reagent-Grade Means. When purchasing chemicals, you may see them identified as "reagent-grade". What this means is that the substance is sufficiently pure that it may be used for physical testing, chemical analysis, or for chemical reactions that require pure chemicals.
What are some examples of chemical reagents?
These are some of the chemical reagents, there are many more. is an alkaline solution of potassium permanganate; used in organic chemistry as a qualitative test for the presence of unsaturation, such as double bonds; used in radical substitution and electrophilic addition reactions in organic chemistry.
Are reagents just a single element?
Some reagents are just a single element. However, most processes require reagents made of chemical compounds. Some of the most common ones are listed below. These are some of the chemical reagents, there are many more.
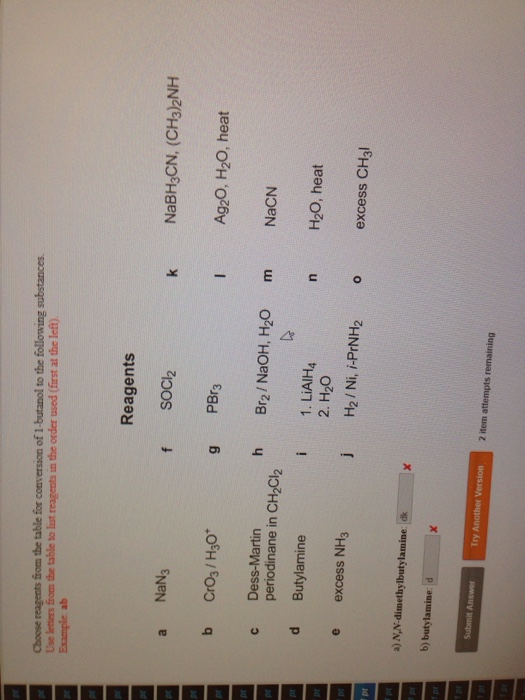
Where are reagents used?
A reagent is an essential component of any chemical reaction. A reagent is a substance or compound that can facilitate a reaction and is used in th...
What are reagents used in chemical reactions?
Reagents are used to confirm the detection of the presence of another substance.
How do reagents work?
Chemical reactions are triggered by reagents. This term includes organic substances that trigger naturally occurring chains of reactions in the bod...
Can reagents be limiting?
Yes, regents can also be limiting. When limiting reagents are consumed completely, the chemical reaction stops. The chemical reaction is dependent...
What is reagent purity?
Reagent grade chemicals are (96-98) % pure, nearly as pure as ACS grade. They are used in the production of food and medicines, as well as in a var...
What is reagent in science?
A reagent is a compound or mixture added to a system to cause a chemical reaction or test if a reaction occurs. A reagent may be used to find out whether or not a specific chemical substance is present by causing a reaction to occur with it.
What Is a Reagent in Chemistry?
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels.
What are some examples of reagents?
Examples of reagents include Grignard reagent, Tollens' reagent, Fehling's reagent, Collins reagent, and Fenton's reagent. However, a substance may be used as a reagent without having the word "reagent" in its name.
What does reagent grade mean?
What Reagent-Grade Means. When purchasing chemicals, you may see them identified as "reagent-grade.". What this means is that the substance is sufficiently pure to be used for physical testing, chemical analysis, or for chemical reactions that require pure chemicals. The standards required for a chemical to meet reagent-grade quality are determined ...
Is a catalyst a reagent or a reactant?
The term reagent is often used in place of reactant, however, a reagent may not necessarily be consumed in a reaction as a reactant would be. For example, a catalyst is a reagent but is not consumed in the reaction. A solvent often is involved in a chemical reaction but it's considered a reagent, not a reactant.
What is a reagent?
Reagents are "substances or compounds that are added to a system in order to bring about a chemical reaction or are added to see if a reaction occurs." Some reagents are just a single element. However, most processes require reagents made of chemical compounds. Some of the most common ones are listed below. These are some of the chemical reagents , there are many more.
What are the simplest reagent compounds?
Reagent Compounds. Name. General Description. Acetic acid. an organic acid; is one of the simplest carboxylic acids. Acetone. an organic compound; simplest example of the ketones. Acetylene. a hydrocarbon and the simplest alkyne; widely used as a fuel and chemical building block.
What is the compound used in NMR?
organic compound; often used as CHCl3 (deuterated chloroform) as a solvent for NMR spectroscopy and as a general solvent. Chromic acid. a strong and corrosive oxidising agent; an intermediate in chromium plating. Chromium trioxide. the acidic anhydride of chromic acid; mainly used in chrome-plating. Collins reagent.
What is the name of the base used in organic synthesis?
in organic synthesis, used for the oxidation of aldehydes to carboxylic acids. Sodium hydride. a strong base used in organic synthesis. Sodium hydroxide. strong base with many industrial uses; in the laboratory, used with acids to produce the corresponding salt, also used as an electrolyte. Sodium hypochlorite.
What is the name of the compound used to oxidize alkenes?
Osmium tetroxide. in organic synthesis, is widely used to oxidise alkenes to the vicinal diols. Oxalyl chloride. used in organic synthesis for the preparation of acid chlorides from the corresponding carboxylic acids.
What is the oxidizing agent used to determine the total oxidisable organic material in an aqueous?
Potassium permanganate. a strong oxidizing agent; can be used to quantitatively determine the total oxidisable organic material in an aqueous sample; a reagent for the synthesis of organic compounds. Pyridinium chlorochromate. used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones.
What is Fenton's reagent?
Fenton's reagent. a solution of hydrogen peroxide and an iron catalyst that is used to oxidize contaminants or waste waters. Formaldehyde. the simplest aldehyde; an important precursor to many other chemical compounds, such as polymers and polyfunctional alcohols.