Which are called representative elements?
Jan 02, 2013 · In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements.
What are the properties of representative elements?
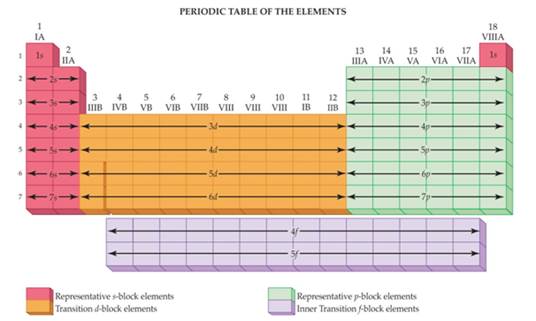
The Representative Elements are those elements within the first two families (Groups I and II on the far left) and the last six families or groups (on the right) of the Periodic Table. The Transition Metals are the elements in those Groups within the middle of the Table.
What are representative and transition elements?
Feb 26, 2015 · The representative elements include eight groups, including the most abundant elements on the planet, not to mention the calcium for your bones, the potassium for your leg cramps, the iodine for ...
What does representative element mean?
Feb 26, 2015 · A representative element is one that has incomplete outermost shells and is reactive. The representative elements in a given group show similar chemical properties.

What are representative elements examples?
Representative Elements ListHydrogen.Lithium.Sodium.Potassium.Rubidium.Caesium.Francium.Sep 22, 2021
What are representative elements Why are they called so?
They are called as representative elements because their outer shells are not completely filled with electrons and The elements get the nearest inert gas configuration by losing or gaining or sharing of electrons. They are chemically active.May 18, 2021
What are the four representative elements?
The elements in the periodic table are arranged into four blocks: s, p, d, and f. The elements present in s- and p-blocks are collectively known as representative elements or main group elements.
How do you identify representative elements?
Answer: The elements within the first two families (Groups I and II on the far left) and the last six families or groups (on the right) of the Periodic Table are known as representative elements. S-lock and P-block elements come under the category of representative elements.
What is another name for representative elements?
Another name for the representative elements are the Group A elements or the main group elements.
Is promethium a representative element or transition?
The period 6 inner transition metals (lanthanides) are cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu).
Why are s and p-block elements called representative elements?
- Both the s and p- block elements are collectively called as the representative elements. It is so because they are chemically very reactive metals and nonmetals and can easily attain the nearest noble gas configuration by losing or gaining the electrons from their valence shell.
Why are representative elements called representative elements?
Representative elements are called representative elements because they are true to the general properties expected of the group they belong to; th...
What is a representative element?
A representative element is one that has incomplete outermost shells and is reactive. The representative elements in a given group show similar che...
What blocks make up the representative elements?
The s block and p block of the periodic table make up the representative elements. These blocks are on the left and right of the periodic table.
What are representative elements?
The Representative Elements are those elements within the first two families (Groups I and II on the far left) and the last six families or groups (on the right) of the Periodic Table. The Transition Metals are the elements in those Groups within the middle of the Table.
What is the difference between transition elements and representative elements?
The difference between representative elements and transition elements is that, representative elements are the chemical elements in the group 1, group 2 and in the groups from 13 to 18 whereas transition elements are chemical elements in group 3 to group 12 including Lanthanides and Actinides.
What are the main groups of elements?
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements ) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements. What is the difference between representative ...
How many groups of elements are there in the representative elements?
The representative elements include eight groups, including the most abundant elements on the planet, not to mention the calcium for your bones, the potassium for your leg cramps, the iodine for Fluffy. . . well, you get the idea. Learning Outcomes. After you have finished this lesson you should be able to:
How does the periodic table organize elements?
The periodic table organizes elements based on similar characteristics. Each group or family has the same number of valence electrons, which gives them similar properties, such as reactivity (even if they do look completely different).
Why do alkali metals have only one valence electron?
You might have guessed it already, but the alkali metals have one valence electron, which makes them quite reactive (think: explosion)! In fact, they don't even exist in nature in their pure, elemental form because they quickly react, or combine, with another element to create something new.
What element group has 6 valence electrons?
Let's check out group 6A, or the chalcogens. This group of elements varies greatly. For example, it contains oxygen, a colorless gas vital to your survival, as well as polonium a radioactive metal that can kill you. Although there is a lot of variation amongst elements in this group, they all have six valence electrons.
How many valence electrons does group 3A have?
Even though each group varies greatly, you can take comfort in knowing that group 3A has three valence electrons, group 4A has four valence electrons, and group 5A has five valence electrons.
Which group of gases has eight valence electrons?
Our tour will conclude with group 8A, or the noble gases. With the exception of helium (which has two), this group has eight valence electrons. This makes noble gases extremely stable, meaning they aren't likely to react with other elements.
What is the periodic table?
The periodic table is a chart that groups elements together based on similar characteristics and atomic structures. The Periodic Table. You'll notice that the symbols are used to save room. For example, instead of aluminum, the periodic table says Al.
Why Are They Called Representative Elements?
The representative elements have other names as well. Initially, eight groups with A and B sub-groups in each divided the elements in the periodic table. Group 1 is 1A, group 2, IIA. The ten groups in the middle, after calcium, fall in groups IIIB, IVB till VIIIB, and then as IB and IIB.
Groups in the Periodic Table Definition
The definition of a group in the periodic table is that it is a vertical column containing chemically similar elements clubbed together.
Representative Metals
Representative metals are members of s block and parts of p block of the table Elements of group 1, (except hydrogen) 2, 13, (except boron) tin and lead of group 14, and bismuth of group 15 are all representative metals. The metals in groups 13, 14, and 15 are not as strong metals as those in groups 1 and 2.
What are representative elements?
The representative elements are those found in the first two groups of the periodic table and in groups 13 through 18. They are also referred to as the main-group elements and comprise all known elements, except the transition metals located in the center of the periodic table. Elements are organized in the periodic table according ...
How are elements organized in the periodic table?
Elements are organized in the periodic table according to their physical characteristics and chemical behaviors. The representative elements in group one are called the alkali metals, and they each possess only one valence electron in their outermost electron shell. The alkali metals are soft, lustrous metals that are highly conductive.
What metals react with water?
From calcium downward, the alkaline earth metals react with room-temperature water, but the reaction is slower and not as violent as a reaction with a group one alkali metal. Many of the representative elements on the right side of the periodic table are nonmetals. The nonmetals in group 18 are called the noble gases, ...
What are the nonmetals in group 18?
The nonmetals in group 18 are called the noble gases, and they mostly do not form bonds with other elements because their electron configurations are stable on their own, although there are exceptions. ADVERTISEMENT.
Which group of metals are harder than the alkali metals in group one?
Group two representative elements are called the alkaline earth metals. These elements are harder than the alkali metals in group one.
Does magnesium react with water?
The extent to which the alkaline earth metals react with water increases while moving down the periodic table. Beryllium, for example, is unable to react with water at all, while magnesium reacts with steam, but not water in the liquid phase.