
What are the types of energy for kids?
energy
- Potential and Kinetic Energy. Each of the different forms of energy can be described as either potential energy or kinetic energy.
- Mechanical Energy. ...
- Heat Energy (Thermal Energy) All substances are made up of particles, or bits, called molecules. ...
- Light Energy. ...
- Sound Energy. ...
- Electrical Energy. ...
- Chemical Energy. ...
- Nuclear Energy. ...
What are some interesting facts about electrons?
What Are Some Interesting Facts About Electrons? Electrons have a negative charge and are found outside the nucleus of an atom. The number of electrons is always the same as the number of protons and they can be found in everything from hydrogen to iron. An arrangement of electrons around an atom’s nucleus is sometimes called an electron cloud.
What is molecule definition for kids?
Introduction A molecule is the smallest unit of a substance that has all the properties of that substance. For instance, a water molecule is the smallest unit that is still water. A water molecule can be divided into tiny parts called atoms. This produces two hydrogen atoms and one oxygen atom.
What is atom -basics for kids?
Science >> Chemistry for Kids The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms fit together with other atoms to make up matter. It takes a lot of atoms to make up anything.
See more
What is an electron simple Definition?
An electron is a negatively charged subatomic particle that can be either bound to an atom or free (not bound). An electron that is bound to an atom is one of the three primary types of particles within the atom -- the other two are protons and neutrons. Together, electrons, protons and neutrons form an atom's nucleus.
What does an electron do for kids?
An atom is the very small unit found inside an element. Inside those are tiny, charged particles called electrons that move like planets orbiting around the sun. Electrons have a lot of energy, they can create electricity in a flow of electrons called an electrical current. They can also bond atoms together.
What is a proton kid Definition?
Kids Definition of proton : a very small particle that exists in the nucleus of every atom and has a positive charge of electricity. proton. noun.
What is an atom Definition for kids?
Atoms are the smallest building blocks of matter and make up everything around us. Every atom has a center called a nucleus, which is made of particles called protons and neutrons. Electrons move in electron shells around the nucleus. Atoms can bond to one another to form solids, liquids, or gases.
What is a electron example?
Electrons are the smallest of the particles that make up an atom, and they carry a negative charge. The number of protons and electrons is equal in a neutral atom. The hydrogen atom, for example, has just one electron and one proton. The uranium atom, on the other hand, has 92 protons, and therefore, 92 electrons.
What is a neutron kid Definition?
Kids Definition of neutron : a particle that has a mass nearly equal to that of the proton but no electrical charge and that is present in all atomic nuclei except those of hydrogen.
Where are the electrons?
Where Are Electrons? Unlike protons and neutrons, which are located inside the nucleus at the center of the atom, electrons are found outside the nucleus. Because opposite electric charges attract each other, negative electrons are attracted to the positive nucleus.
What is proton neutron and electron for kids?
Each individual atom is made up of smaller particles—electrons, protons, and neutrons. These are called subatomic particles. At the center of an atom is a nucleus. The nucleus consists of protons and neutrons. Protons carry a positive electrical charge, while neutrons carry no electrical charge.
What are electrons made of?
The Atom Builder Guide to Elementary Particles Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom's nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom's nucleus.
What is an atom 1st grade?
0:003:26What is an Atom? - Structure of an Atom - Atom video for kids - YouTubeYouTubeStart of suggested clipEnd of suggested clipAn atom is made up of three parts protons neutrons and electrons come on let's learn about these oneMoreAn atom is made up of three parts protons neutrons and electrons come on let's learn about these one by one protons a proton is a positively charged particle found within the nucleus of an atom.
What is element for Kids?
An element is a pure substance that is made from a single type of atom. Elements are the building blocks for all the rest of the matter in the world. Examples of elements include iron, oxygen, hydrogen, gold, and helium. Atomic Number. An important number in an element is the atomic number.
What is an ion for Kids?
An ion is a particle like an atom that has lost or gained electrons. Because electrons are negative and protons are positive, differing amounts create a charge. Oppositely charged ions attract just like a magnet and form bonds.
What is the function of a electron?
An electron generates an electric field that exerts an attractive force on a particle with a positive charge, such as the proton, and a repulsive force on a particle with a negative charge.
What are 3 facts about electrons?
Electrons are found free in nature (free electrons) and bound within atoms. Electrons are responsible for the negatively-charged component of an atom. In an atom, electrons orbit around the positively-charged atomic nucleus. In solids, electrons are the primary means of conducting current.
What are two important facts about the electron?
electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton.
What is an element KIDS Definition?
Kids Definition of element 1 : any of more than 100 substances that cannot by ordinary chemical means be separated into different substances Gold and carbon are elements. 2 : one of the parts of which something is made up There is an element of risk in surfing.
Are electrons positive or negative?
Because a proton has a positive charge (+) and an electron has a negative charge (-), element atoms are neutral, with all positive charges cancelli...
Who named Electron?
G. Johnstone Stoney invented the term “electron” in 1891 to describe the unit of charge discovered in tests that conveyed electric current through...
Do protons and electrons have the same mass?
Electrons are a sort of negative-charged subatomic particle. Protons and neutrons have about the same mass as electrons, yet they are both signific...
Are protons and electrons equal?
Protons and electrons are in equal proportions in an atom. Because protons and electrons have equal and opposing charges, atoms are generally neutral.
Why do electrons repel each other?
A free electron will flow in the opposite direction of the force lines since it has the opposite charge qualities as a positive charge. As a result...
What is the mass of an electron?
: an elementary particle consisting of a charge of negative electricity equal to about 1.602 × 10−19 coulomb and having a mass when at rest of about 9.109 × 10−31 kilogram or about ¹/₁₈₃₆ that of a proton.
How many electron volts does a neutrinos have in 2021?
— Anna Blaustein, Scientific American, 28 June 2021 For instance, the neutrinos investigated by the MINOS experiment average about six billion electron volts of energy.
What is the charge of an electron?
The electron has a negative electric charge . The electron has another property, called spin. Its spin value is 1/2, which makes it a fermion . While most electrons are found in atoms, others move independently in matter, or together as cathode rays in a vacuum. In some superconductors, electrons move in pairs.
When was the electron discovered?
An electron is a very small piece of matter. Its symbol is e−. It was discovered by J. J.Thomson in 1897.
How many electrons can be in an electron shell?
Each electron shell is given a number 1, 2, 3, and so on, starting from the one closest to the nucleus (the innermost shell). Each shell can hold up to a certain maximum number of electrons. The distribution of electrons in the various shells is called electronic arrangement (or electronic form or shape).
How are electrons attracted to atoms?
For this reason, electrons are attracted by the protons of atomic nuclei and usually form atoms . An electron has a mass of about 1/1836 times a proton. One way to think about the location of electrons in an atom is to imagine that they orbit at fixed distances from the nucleus. This way, electrons in an atom exist in a number ...
Why do electrons repel each other?
The electromagnetic force is strongest in common situations. Electrons repel (push apart) from each other because they have the same electric charge. Electrons are attracted to protons because they have opposite electric charge. An electron has an electric field, which describes these forces. The electricity that powers televisions, motors, mobile phones, and many other things is actually many electrons moving through wires or other conductors .
What is the uncertainty principle of electron cloud?
The uncertainty principle means a person cannot know an electron's position and energy level at the same time. These potential states form a cloud around the atom.
How to observe electron interactions?
In laboratory conditions, the interactions of individual electrons can be observed by means of particle detectors, which allow measurement of specific properties such as energy, spin and charge. In one instance a Penning trap was used to contain a single electron for 10 months. The magnetic moment of the electron was measured to a precision of eleven digits, which, in 1980, was a greater accuracy than for any other physical constant.
What are Electrons?
Inside an atom, the very small unit found inside an element, there are even tinier particles that move like planets orbiting around the sun. These particles move at such an incredible speed that you cannot even see them under a microscope.
What are electrons charged with?
We call them electrons, and they are particles charged up with tons of energy. They are so small, but they contain the same amount of energy as a particle that is 1,000 times its size. No wonder they move so fast!
How to see electrical current in action?
Want to see an electrical current in action? Rub a balloon against your head and watch the atoms in your hair try and stick to the atoms in the balloon. Their electrons are attracted to each other!
What happens when electrons collide with each other?
It's like a super speedy game of hot potato! As these electrons get passed around so rapidly, they collide with each other and create the electricity that you use when you turn on a light switch, plug in a charger, or watch TV.
How many electrons does an atom have?
Some types of atoms have more electrons than others. For example, an oxygen atom, found in the air, has only 8 electrons. Meanwhile, a copper atom, found in pennies and pipes in your house, have 29 electrons.
Why are electrons important?
Well, take a closer look at that word and you will see 'electron.'. Electrons are important for so many amazing things that happen around us, including electricity. This lesson focuses on the nature of electrons, where they are found, and how they work. Updated: 02/17/2020.
What holds atoms together?
Everywhere you look, atoms are bound together. For example, water is made up of hydrogen and oxygen. What holds these atoms together? Electrons, of course!
What are Electrons?
Electrons are subatomic particles that hold an elementary charge of magnitude -1. The charge of an electron is equal in magnitude to the charge held by a proton (but has an opposite sign). Therefore, electrically neutral atoms/molecules must have an equal number of electrons and protons. Although the magnitude of the charges held by protons and electrons are the same, the size and mass of an electron are much smaller than that of a proton (the mass of an electron is roughly 1/1836 the mass of a proton).
Who invented the term "electron"?
G. Johnstone Stoney invented the term “electron” in 1891 to describe the unit of charge discovered in tests that conveyed electric current through chemicals. J.J. Thomson’s Cambridge classmate Joseph Larmor used the phrase in this context.
Why are protons and electrons in equal proportions?
Protons and electrons are in equal proportions in an atom. Because protons and electrons have equal and opposing charges, atoms are generally neutral.
Why are element atoms neutral?
Because a proton has a positive charge (+) and an electron has a negative charge (-), element atoms are neutral, with all positive charges cancelling out all negative charges. The number of protons, neutrons, and electrons in an atom varies from one to the next.
What is the nucleus of an atom?
The nucleus contains the protons and the neutrons. Considering the solar system, it has been observed that the sun is at its centre, and the planets revolve around it. Similarly, in an atom, the nucleus is at the centre, and the electrons revolve around the nucleus. Electron structure.
Which subatomic particle has the same mass as an electron?
Electrons are a sort of negative-charged subatomic particle. Protons and neutrons have about the same mass as electrons, yet they are both significantly more massive (approximately 2,000 times as massive as an electron). A proton’s positive charge is the same magnitude as an electron’s negative charge.
Why is the mass of an electron negligible?
This is because matter behaves differently at the quantum scale. For example, the uncertainty associated ...
What is the structure of electrons in an atom called?
The detailed structural arrangement of electrons within an atom is referred to as the electronic configuration of the atom.
What is the mass of an electron?
The rest mass of the electron is 9.1093837015 × 10 −31 kg, which is only 1/1,836 the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
How do electrons move around an atom?
Within any given atom, electrons move about the nucleus in an orderly arrangement of orbitals, the attraction between electrons and nucleus overcoming repulsion among the electrons that would otherwise cause them to fly apart. These orbitals are organized in concentric shells proceeding outward from the nucleus with an increasing number of subshells. The electrons in orbitals closest to the nucleus are held most tightly; those in the outermost orbitals are shielded by intervening electrons and are the most loosely held by the nucleus. As the electrons move about within this structure, they form a diffuse cloud of negative charge that occupies nearly the entire volume of the atom. The detailed structural arrangement of electrons within an atom is referred to as the electronic configuration of the atom. The electronic configuration determines not only the size of an individual atom but also the chemical nature of the atom. The classification of elements within groups of similar elements in the periodic table, for example, is based on the similarity in their electron structures.
How are electrons held in orbitals?
These orbitals are organized in concentric shells proceeding outward from the nucleus with an increasing number of subshells. The electrons in orbitals closest to the nucleus are held most tightly; those in the outermost orbitals are shielded by intervening electrons and are the most loosely held by the nucleus.
How are electrons bound to the nucleus?
Under ordinary conditions electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus.
What is a lepton?
A lepton is a subatomic particle that reacts only by the electromagnetic, weak, and gravitational forces; it does not respond to the short-range strong force that acts between quarks and binds protons and neutrons in the atomic nucleus. Get a Britannica Premium subscription and gain access to exclusive content.
When were electrons discovered?
The electron was discovered in 1897 by the English physicist J.J. Thomson during investigations of cathode rays. His discovery of electrons, which he initially called corpuscles, played a pivotal role in revolutionizing knowledge of atomic structure. Under ordinary conditions electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Any atom, however, may have more or fewer electrons than positive charges and thus be negatively or positively charged as a whole; these charged atoms are known as ions. Not all electrons are associated with atoms; some occur in a free state with ions in the form of matter known as plasma.
What is an electron?
Anne Marie Helmenstine, Ph.D. Updated July 24, 2019. An electron is a stable negatively charged component of an atom. Electrons exist outside of and surrounding the atom nucleus. Each electron carries one unit of negative charge (1.602 x 10 -19 coulomb) and has a small mass as compared with that of a neutron or proton.
Where did the term "electron" come from?
The words "electron" and "electricity" trace their origins to the ancient Greeks. The ancient Greek word for amber was elektron.
How much does an electron weigh?
Electrons are much less massive than protons or neutrons. The mass of an electron is 9.10938 x 10 -31 kg. This is about 1/1836 the mass of a proton. In solids, electrons are the primary means of conducting current (since protons are larger, typically bound to a nucleus, and thus more difficult to move).
Why do we have chemical bonds?
Chemical bonds are the result of transfers or sharing of electrons between atoms.
What happens when there are more electrons than positive charges?
If there are more electrons than positive charges, a material is said to be negatively charged. If there is an excess of protons, the object is considered to be positively charged. If the number of electrons and protons is balanced, a material is said to be electrically neutral. Electrons can exist free in a vacuum.
Why are electrons considered elementary particles?
Electrons are considered to be a type of elementary particle because they are not made up of smaller components. They are a type of particle belonging to the lepton family and have the smallest mass of any charged lepton or other charged particle. In quantum mechanics, electrons are considered to be identical to each other because no intrinsic ...
What is the symbol for an electron?
A common symbol for an electron is e -. The electron's antiparticle, which carries a positive electric charge, is called a positron or antielectron and is denoted using the symbol β -. When an electron and a positron collide, both particles are annihilated and gamma rays are released.
Where do electrons spin?
The electron is a negatively charged particle that spins around the outside of the nucleus. Electrons spin so fast around the nucleus, scientists can never be 100% sure where they are located, but scientists can make estimates of where electrons should be.
Which atom has a single proton?
The Proton. The proton is a positively charged particle that is located at the center of the atom in the nucleus. The hydrogen atom is unique in that it only has a single proton and no neutron in its nucleus. The Electron. The electron is a negatively charged particle that spins around the outside of the nucleus.
What is the nucleus of an atom?
But the nucleus is very hard to split, meaning most atoms are around for a long time. At the center of the atom is the nucleus. The nucleus is made up of the protons and neutrons. The electrons spin in orbits around the outside of the nucleus. The proton is a positively charged particle that is located at the center of the atom in the nucleus.
What is the charge of an atom?
If there are the same number of electrons and protons in an atom, then the atom is said to have a neutral charge . Electrons are attracted to the nucleus by the positive charge of the protons. Electrons are much smaller than neutrons and protons. About 1800 times smaller!
How many atoms are there in an element?
Each different kind of atom makes up an element. There are 92 natural elements and up to 118 when you count in man-made elements. Atoms last a long time, in most cases forever. They can change and undergo chemical reactions, sharing electrons with other atoms.
Does a neutron have a charge?
The neutron doesn't have any charge. The number of neutrons affects the mass and the radioactivity of the atom. Quark - The quark is a really small particle that makes up neutrons and protons. Quarks are nearly impossible to detect and it's only recently that scientists figured out they existed.
How do electrons build up in an object?
Most objects have a balance of positive and negative charges, so they are considered neutral. This means that they do not push or pull on each other electrically. However, sometimes electrons can build up in an object. Two such objects can push or pull on each other because they are no longer neutral. This push or pull from extra electrons is called static electricity. Static electricity can cause interesting effects, such as sparks or lightning bolts, when it is released. Sometimes the extra electrons build up by rubbing one object against another. For example, when one rubs a balloon against one’s hair, electrons move from the balloon to the hair. Because the hairs then all have extra electrons, which all have the same kind of charge, they try to fly away from each other and end up sticking into the air like spikes!
What is the term for the push or pull of electrons?
This push or pull from extra electrons is called static electricity. Static electricity can cause interesting effects, such as sparks or lightning bolts, when it is released. Sometimes the extra electrons build up by rubbing one object against another. For example, when one rubs a balloon against one’s hair, electrons move from the balloon to ...
What is the name of the tiny particles that swirl around each other?
Everything in the universe is made of tiny objects called atoms. Each atom has even tinier particles called protons and electrons. These tiny particles swirl around each other continuously. An electron has what is called a negative charge. A proton has a positive charge.
What is electricity in science?
Electricity is the flow of tiny particles called electrons and protons. It can also mean the energy you get when electrons flow from place to place. Electricity can be seen in nature in a bolt of lightning.
Why do electrons move from a balloon to the hair?
For example, when one rubs a balloon against one’s hair, electrons move from the balloon to the hair. Because the hairs then all have extra electrons, which all have the same kind of charge, they try to fly away from each other and end up sticking into the air like spikes!
What is the charge of a proton?
A proton has a positive charge. Positive and negative charges try to pull each other together. However, two positive charges, or two negative charges, will push each other away. Electricity results when electrons are pushed and pulled from atom to atom.
How does electricity flow?
Many moving electrons are called an electric current. A city’s power plant produces a powerful electric current and sends it through wires. The electricity used for lighting, heating, and running appliances is made by machines called generators. Generators cause a current to flow by moving a magnet past a coil of wire, which pushes electrons through the wires of the coil. Wires carrying the current travel to houses and other buildings. More wires connect to the power outlets in rooms. When a person plugs in an iron or another electric device, the current travels into the device. The current then makes the device work. A chemical reaction in a battery can also produce an electric current.
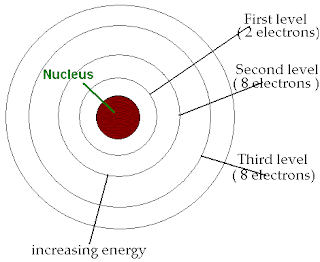
Description
History of Its Discovery
- Electrons have the smallest electrical charge. This electrical charge equals the charge of a proton, but has the opposite sign. For this reason, electrons are attracted by the protons of atomic nuclei and usually form atoms. An electron has a mass of about 1/1836 times a proton. One way to think about the location of electrons in an atom is to imag...
The Electron Cloud Model
Related Pages
- Electrons have the smallest electrical charge. This electrical charge equals the charge of a proton, but has the opposite sign. For this reason, electrons are attracted towards the protons in atomic nuclei. This attraction makes electrons near a nucleus form an atom.An electron has a mass of about 1/1836 times a proton. While most electrons are fou...
Other Websites
- The effects of electrons were known long before it could be explained. The Ancient Greeks knew that rubbing amber against fur attracted small objects. Now we know the rubbing strips off electrons, and that gives an electric charge to the amber.Many physicists worked on the electron. J.J. Thomson proved it existed, in 1897, but another man gave it the name 'electron'.