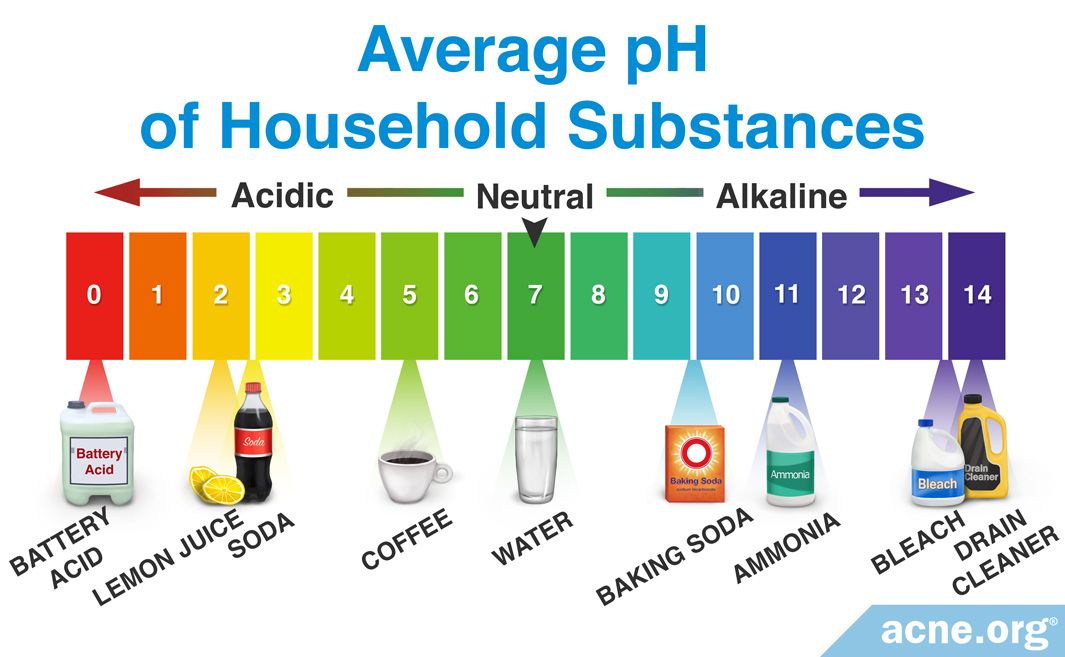
What is an example of something that has a basic pH? Basic substances include things like baking soda, soap, and bleach. Distilled water is a neutral substance. The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is.
What pH level is the most basic?
· What is an example of something that has a basic pH? Basic substances include things like baking soda, soap, and bleach. Distilled water is a neutral substance. The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Click to see full answer. Keeping this in view, what is an example of pH?
What are the basics of pH?
What is an example of something that has a basic pH? Basic substances include things like baking soda, soap, and bleach. Distilled water is a neutral substance. The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. 🤮😖😲 Click to see full answer. Also to know is, what is an example of pH?
What are basic question and answers in pH?
The pH scale, with examples of common solutions and their pH values. Download/View. For commercial use please contact us.
What pH values are basic?
· pH is a measure of how acidic or basic an aqueous solution is. pH usually ranges from 0 (acidic) to 14 (basic). A pH value around 7 is considered neutral. pH is measured using pH paper or a pH meter. Most fruits, vegetables, and body fluids are acidic. While pure water is neutral, natural water may be either acidic or basic. Cleaners tend to be basic.

What is the most basic thing pH?
The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic.
What household items have a basic pH?
Anything with a pH of more than 7 is a base....Explanation:Sodium Bicarbonate - Baking Soda.Soap (A mild base)Oven Cleaner.Drain Cleaner.Toothpaste.Bleach.Ammonia (Sometime found in hair products or cleaning products)Washing Powder.More items...•
What is an example of something that has an acidic pH?
If a solution contains more hydrogen ions than hydroxide ions, it is said to be acidic, and the pH of the solution is less than 7....The pH scale.Increasing pH (Decreasing Acidity)Substances0 (most acidic)Hydrochloric acid (HCl)1Stomach acid2Lemon juice3Cola, beer, vinegar15 more rows
What are 5 common bases?
Here is a list of the most common strong bases.LiOH - lithium hydroxide.NaOH - sodium hydroxide.KOH - potassium hydroxide.RbOH - rubidium hydroxide.CsOH - cesium hydroxide.*Ca(OH)2 - calcium hydroxide.*Sr(OH)2 - strontium hydroxide.*Ba(OH)2 - barium hydroxide.
What items are basic?
Basic substances react to aqueous solutions by accepting protons, giving away electrons, or releasing hydroxide ions....Examples of Everyday BasesDrain cleaner.Laundry detergent.Lubricating grease.Alkaline batteries.Soaps and bath products.Sugar.Baking soda.
Is vinegar acidic or basic?
Vinegar is mildly acidic with a pH of 2–3. Apple cider vinegar is slightly more alkaline than pure vinegar because it contains more alkaline nutrients. However, it's still acidic.
Is milk an acid or base?
Milk — pasteurized, canned, or dry — is an acid-forming food. Its pH level is below neutral at about 6.7 to 6.9. This is because it contains lactic acid. Remember, though, that the exact pH level is less important than whether it's acid-forming or alkaline-forming.
Is dish soap a base or acid?
Dish soap is neither an acid nor base. Ph 7 to 8 (neutral cleaner) when it comes to neutrality mild dish soap usually hits the mark perfectly. This mildness makes dish soap perfect for day to day cleaning.
How to measure pH?
To measure pH, a piece of pH test paper or an indicator stick is dipped into the liquid. The color of the dipped paper/stick is then matched to a color key that comes with the container of pH test paper or indicator sticks. Each color on the key represents a different pH.
What is the equation for pH?
To be more precise, pH is the negative logarithm of the hydrogen ion concentration: pH = −log [H+] The square brackets around the H + automatically mean "concentration" to a chemist. What the equation means is just what we said before: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold.
Is water acidic or basic?
In pure water, there are an equal number of hydrogen ions and hydroxide ions. The solution is neither acidic or basic. An acid is a substance that donates hydrogen ions. Because of this, when an acid is dissolved in water, the balance between hydrogen ions and hydroxide ions is shifted.
What is the difference between acid and base?
Now there are more hydrogen ions than hydroxide ions in the solution. This kind of solution is acidic. A base is a substance that accepts hydrogen ions.
What is the pH scale?
In order to deal with these large numbers more easily, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14.
Is pH a logarithm?
It is not coincidence, it is logarithms! To be more precise, pH is the negative logarithm of the hydrogen ion concentration: pH = −log [H+] The square brackets around the H + automatically mean "concentration" to a chemist.
What is the pH of pure water?
What the equation means is just what we said before: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold. Pure water has a neutral pH of 7. pH values lower than 7 are acidic, and pH values higher than 7 are alkaline (basic).
What is the pH of a chemical?
Key Takeaways: pH of Common Chemicals 1 pH is a measure of how acidic or basic an aqueous solution is. pH usually ranges from 0 (acidic) to 14 (basic). A pH value around 7 is considered neutral. 2 pH is measured using pH paper or a pH meter. 3 Most fruits, vegetables, and body fluids are acidic. While pure water is neutral, natural water may be either acidic or basic. Cleaners tend to be basic.
What is the pH of a solution?
pH is a measure of how acidic or basic an aqueous solution is. pH usually ranges from 0 (acidic) to 14 (basic). A pH value around 7 is considered neutral. pH is measured using pH paper or a pH meter. Most fruits, vegetables, and body fluids are acidic.
How to test pH of a substance?
There are multiple ways to test the pH of substances. The simplest method is to use pH paper test strips. You can make these yourself using coffee filters and cabbage juice, use Litmus paper, or other test strips. The color of the test strips corresponds to a pH range.
What is the pH of an aqueous solution?
pH is a measure of how acidic or basic an aqueous solution is. pH usually ranges from 0 (acidic) to 14 (basic). A pH value around 7 is considered neutral.
Is milk acidic or neutral?
Milk is often considered to be neutral, since it's only slightly acidic. Milk becomes more acidic over time. The pH of urine and saliva is slightly acidic, around a pH of 6. Human skin, hair, and nails tends to have a pH around 5. 0 - Hydrochloric Acid (HCl) 1.0 - Battery Acid (H 2 SO 4 sulfuric acid) and stomach acid.
Why is distilled water acidic?
Distilled water tends to be slightly acidic because of dissolved carbon dioxide and other gases. Pure water is nearly neutral, but rain water tends to be slightly acidic. Natural water rich in minerals tends to be alkaline or basic.
What is the pH of a plant?
Most plants prefer a pH between 5.5 and 7.5. Stomach acid contains hydrochloric acid and other substances and has a pH value of 1.2. While pure water free of undissolved gases is neutral, not much else is. However, buffer solutions may be prepared to maintain a pH near 7.
What does pH mean in water?
The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
What does a pH of 7 mean?
The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic.
What is the significance of pH in water?
Since pH can be affected by chemicals in the water, pH is an important indicator of water that is changing chemically. pH is reported in "logarithmic units". Each number represents a 10-fold change in the acidity/basicness of the water.
How to measure pH?
One easy way that you can measure pH is with a strip of litmus paper. When you touch a strip of litmus paper to something, the paper changes color depending on whether the substance is acidic or basic. If the paper turns red, the substance is acidic, and if it turns blue, the substance is basic.
What is the pH scale?
The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7.
What is the pH of pure water?
As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic.
What is the pH of a solution of a strong acid?
When a base, or alkali, is dissolved in water, the pH will be greater than 7. A solution of a strong acid, such as hydrochloric acid, at concentration 1 mol dm −3 has a pH of 0. A solution of a strong alkali, such as sodium hydroxide, at concentration 1 mol dm −3, has a pH of 14.
What is the pH scale?
The pH scale is logarithmic and inversely indicates the concentration of hydrogen ions in the solution. This is because the formula used to calculate pH approximates the negative of the base 10 logarithm of the molar concentration of hydrogen ions in the solution.
What is the pH of a neutral pH scale?
The neutral value of the pH depends on the temperature – being lower than 7 if the temperature increases. The pH value can be less than 0 for very strong acids, or greater than 14 for very strong bases. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.
When was pH first used?
History. The concept of pH was first introduced by the Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909 and revised to the modern pH in 1924 to accommodate definitions and measurements in terms of electrochemical cells.
When was the pH concept invented?
The concept of pH was first introduced by the Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909 and revised to the modern pH in 1924 to accommodate definitions and measurements in terms of electrochemical cells.
What is pOH in water?
pOH is sometimes used as a measure of the concentration of hydroxide ions, OH −. pOH values are derived from pH measurements. The concentration of hydroxide ions in water is related to the concentration of hydrogen ions by
Can hydrogen ion concentrations be measured in non-aqueous solvents?
Hydrogen ion concentrations (activities) can be measured in non-aqueous solvents. pH values based on these measurements belong to a different scale from aqueous pH values, because activities relate to different standard states. Hydrogen ion activity, aH+, can be defined as:

What Is An Acid Or A Base?
What Is Ph?
- Acidity and alkalinity are measured with a logarithmic scale called pH. Here is why: a strongly acidic solution can have one hundred million million, or one hundred trillion (100,000,000,000,000) times more hydrogen ions than a strongly basic solution! The flip side, of course, is that a strongly basic solution can have 100,000,000,000,000 times mo...
How Do You Measure Ph?
- The pH of a liquid or solution is often an important piece of information in science. Measuring pH can be done simply and quickly using pH test paper, pH indicator sticks, or a pH meter. pH test paper and indicator sticks are pieces of paper or stiffer sticks that contain pH indicators(chemicals that change color depending on how acidic or basic a solution is). To mea…
Bibliography
- For more information about acids, bases, and the pH scale, try this reference: 1. Khan Academy. (2009, September 7). Arrhenius acids and bases. Retrieved July 15, 2021.