
10 Law of Conservation of Mass Examples in Real Life
- Burning wood. In campsites, it’s popular for people to start fires using wood for cooking or warming themselves. ...
- Brewing Beer. The brewing process for beer shows that mass is conserved. ...
- Photosynthesis. ...
- Disinfectant decomposition reactions. ...
- Synthesis reactions. ...
- Lit Candles. ...
- The vinegar and baking soda experiment. ...
- Algebraic equations. ...
Full Answer
What does the law of Conservation of matter tell us?
The Law of Conservation of Matter says that the amount of matter stays the same, even when matter changes form. Sometimes it may seem that matter disappears during a science experiment, but this law tells us that matter cannot magically appear or disappear, it simply changes from one form to another.
What are some examples of the law of conservation?
- The law of conservation of energy can be seen in everyday examples of energy transfer.
- The law of conservation of energy is a physics concept.
- The law of conservation of energy is a science law that says energy cannot be created or destroyed.
- An example of the law of conservation of energy is that water can produce electricity.
What does conservation of matter stand for?
What Does Conservation of Matter Mean? The law of conservation of matter is a fundamental principle of classical physics that states that matter cannot be created nor destroyed in any isolated system, but can only be converted from one form to another.
How can the law of Conservation of matter be demonstrated?
When balancing chemical equations, the law of conservation of mass is also demonstrated because the total number of atoms that goes into the reaction must be produced. So if 14 atoms are on the reactant side, then 14 atoms must be on the product side. Equations often require balancing to correctly demonstrate the law of conservation of mass.
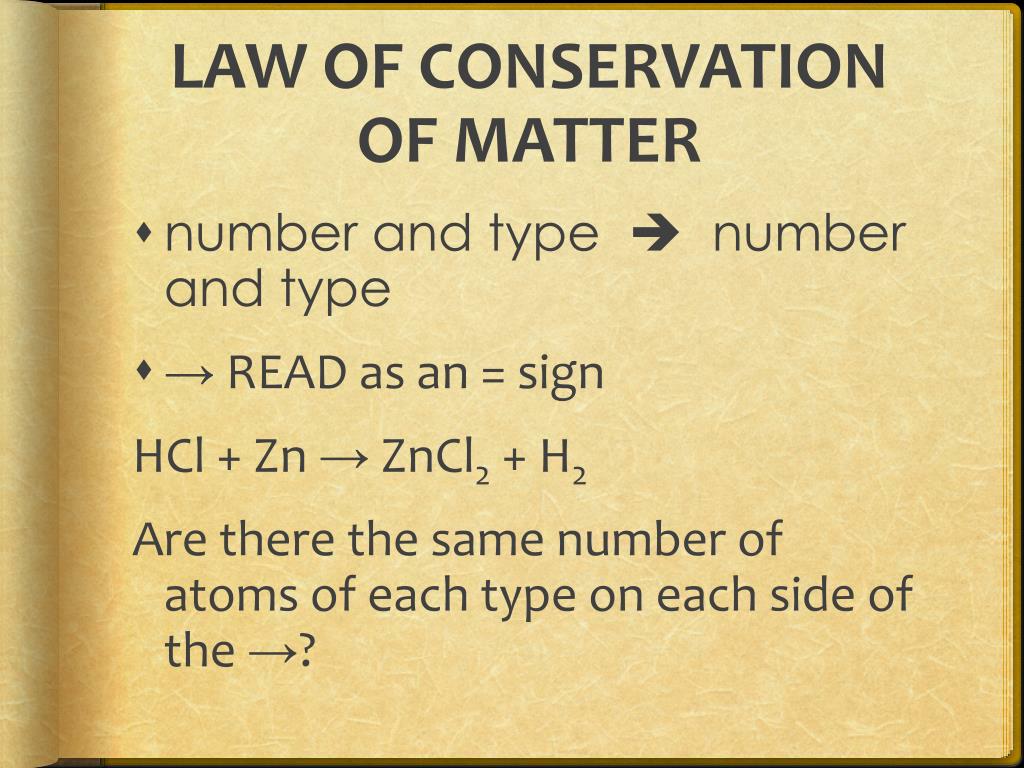
What is an example of conservation of matter?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
What are 3 examples of conservation of matter?
CONSERVATION OF MATTER EXAMPLESWhen something burns up, the matter does not vanish. The materials simply turn into gases you cannot see.When you bake, food seems to magically get larger. Expanding air bubbles caused the baked treats to expand, but more matter was not formed.Candles change form when they are burned.
What is conservation matter?
According to the law of conservation of matter, matter is neither created nor destroyed, so we must have the same number and kind of atoms after the chemical change as were present before the chemical change.
Which is the best example of law of conservation of mass?
A sample of air increases in volume when heated at constant pressure but its mass remains unaltered.
What are some real life examples of the law of conservation of mass?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
What is the law of conservation of matter quizlet?
Law of Conservation of Matter. States that, during a chemical reaction, matter cannot be created or destroyed. Even though the matter may change from one form to another, the same number of atoms exists before and after the changes take place. reactant.
Why is the law of conservation of matter important?
Why is the law of conservation of matter so important? The formulation of this law was crucial in progress from alchemy to the modern natural science of chemistry. Conservation laws are fundamental to our understanding of the physical world in that they describe which processes can or cannot occur in nature.
Why do we need to conserve matter and energy?
Energy needs to be conserved not only to cut costs but also to preserve the resources for longer use. As of today, most of the energy is generated from coal powered power plants. These plants do generate energy but also pollute the environment by emitting harmful gases in the atmosphere.
Which is not an example of matter?
Non-matter includes the light from a torch, the heat from a fire, and the sound of a police siren. You cannot hold, taste, or smell these things. They are not types of matter, but forms of energy. Everything that exists can be classed as either a type of matter or a form of energy.
What are 5 examples of conservation of energy?
1 AnswerA pendulum: As the pendulum swings down: ... A ball tossed up in the air: During the throw: ... A skier slides down a hill: gravitational potential energy of the skier → ... A compressed spring launches a ball in a pinball game: Elastic potential energy of the spring → ... Inside of a nuclear power plant:
Which of the following is are an example of conservation of?
The Biosphere reserve is the example of in conservation.
Which is the best example of law of conservation of energy?
What is the best example of the law of conservation of energy? If a pendulum is one meter off the ground at one end of its swing, it can never be more than one meter (The law of conservation of energy states that energy cannot be created or destroyed.
What is conservation of mass?
The law of conservation of mass states that, during processes like chemical reactions, matter can neither be created nor destroyed.
What does the law of conservation of matter state?
The law of conservation of matter states that no matter can ever be created or destroyed. Chemical reactions simply rearrange atoms to form new com...
What is an example of conservation of matter?
A common example of the law of conservation of matter is the reaction of baking soda and vinegar. At first glance, the reaction seems to finish wit...
What is matter in science?
Matter is anything that has mass and takes up space. It includes molecules, atoms, fundamental particles, and any substance that these particles make up. Matter can change form through physical and chemical changes, but through any of these changes matter is conserved. The same amount of matter exists before and after the change—none is created ...
Why is matter important to the universe?
Matter makes up everything visible in the known universe, from porta-potties to supernovas. And because matter is never created or destroyed, it cycles through our world. Atoms that were in a dinosaur millions of years ago—and in a star billions of years before that—may be inside you today.
How do animals use chemical energy?
When animals in and around the stream eat these plants, their bodies use the stored chemical energy to power their cells and move around. They use the nutrients in their food to grow and repair their bodies—the atoms for new cells must come from somewhere. Any food that enters an animal’s body must either leave its body or become part of it; no atoms are destroyed or created.
What happens when water flows through a canyon?
As the liquid water flows through the canyon, it may evaporate (another physical change) into water vapor. Water gives a very clear example of how matter cycles through our world, frequently changing form but never disappearing. Next, consider the plants and algae living in and along the stream.
Does matter change in the rock?
This is a physical change for the rock, but with the right conditions the rock may chemically change too. In either case, the matter in the rock is conserved. The bottom line is: Matter cycles through the universe in many different forms. In any physical or chemical change, matter doesn’t appear or disappear.
Is matter conserved in chemical changes?
In chemical changes, just as in physical changes, matter is conserved. The difference in this case is that the substances before and after the change have different physical and chemical properties. Hydrogen and oxygen are gases at standard temperature and pressure, whereas water is a colorless, odorless liquid.
Law of Conservation of Matter Definition
The law of conservation of matter, also know as "the law of conservation of mass," states that matter can neither be created nor destroyed. The law's modern form stems from Antoine Lavoisier's work with chemical reactions in the late 18th century. It replaced the phlogiston theory, which stated that mass was destroyed during combustion processes.
What is Conservation of Matter?
Matter is defined as physical material that occupies space and possesses mass. The phlogiston theory, which had a very long history, was built upon the observation that fuel loses mass as it burns. This is a correct observation: after you burn a piece of wood, the wood has less mass than it did prior to burning.
What Are the Implications of This Law?
For chemists, the implications of this law are many. First, the law allowed for chemical reactions to be fully understood and fully quantified. Previously ignored chemical substances (like carbon dioxide being released from a fire) were studied and measured.
What is the law of conservetion?
The law of conservetion of matter is a general law in physics and chemistry applicable for any system closed to all transfers of matter and energy. According to this law the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves. Skip to content.
Why is conservation law important?
Conservation laws are fundamental to our understanding of the physical world in that they describe which processes can or cannot occur in nature.
What is the law of mass?
The law requires that during any nuclear reaction, radioactive decay or chemical reaction in an isolated system, the total mass of the reactants or starting materials must be equal to the mass of the products.
How to find the equivalence of mass and energy?
Equivalenceof the mass and energy is described by Einstein’s famous formula E = mc2. In words, energyequals massmultiplied by the speed of light squared. Because the speed of light is a very large number, the formula implies that any small amount of matter contains a very large amount of energy.
Is the rest mass of an atomic nucleus smaller than the sum of the rest masses of its constituents?
It was found the rest mass an atomic nucleus is measurably smaller than the sum of the rest masses of its constituent protons, neutrons and electrons. For example, the pair productionphenomenon is associated with creation and destruction of matter in one reaction. The Law of Conservation of Matter – Conservation of Mass.
Can matter be destroyed?
According to classical physics matter cannot be destructed. But in special theory of relativity, certain types of matter may be created or destroyed, but in all of these processes, the mass and energy associated with such matter remains unchanged in quantity.
Is matter created or destroyed?
In the special theory of relativity, certain types of matter may be created or destroyed. Still, the mass and energy associated with such matter remain unchanged in quantity in all of these processes.
Who tested the conservation of matter?
Lavoisier’s experiments marked the first time someone clearly tested this idea of the conservation of matter by measuring the masses of materials both before and after they underwent a chemical reaction.
Why is conservation of mass important?
This law is important in chemistry, particularly when combining different materials and testing the reactions between them. In chemistry, the law of conservation of mass states that the mass of the products (the chemical substances created by a chemical reaction) will always equal the mass of the reactants ...
What Is the Law of Conservation of Mass?
First off, exactly what is the law of conservation of mass? This law states that in a closed system, matter can neither be created nor destroyed—it can only change form.
What Is the History Behind the Law of Conservation of Mass?
Though many people, including the ancient Greeks, laid the scientific groundwork necessary for the discovery of the law of conservation of mass, it is French chemist Antoine Lavoisier (1743-1794) who is most often credited as its discoverer. This is also why the law is occasionally called Lavoisier’s law.
Why was the law of conservation of mass important to the field of chemistry?
Ultimately, the discovery of the law of conservation of mass was immensely significant to the field of chemistry because it proved that matter wasn’t simply disappearing (as it appeared to be) but was rather changing form into another substance of equal mass.
What is the law of mass in an isolated system?
Put differently, the amount, or mass, of matter in an isolated system will always be constant regardless of any chemical reactions or physical changes that take place. (Note that an isolated or closed system is one that does not interact with its environment.) This law is important in chemistry, particularly when combining different materials ...
Who discovered that matter is always conserved in a chemical reaction?
Lavoisier lookin' proud of his discovery. In the late 1700s, Lavoisier proved through experimentation that the total mass does not change in a chemical reaction, leading him to declare that matter is always conserved in a chemical reaction. Lavoisier’ s experiments marked the first time someone clearly tested this idea of the conservation ...
What are some examples of conservation of mass?
All types of chemical reactions,nuclear reactions are example of conservation of mass which is a matter within certain space size.You can check that by looking such reactions in any book of chemistry or nuclear physics.
Why are chemical reactions considered to be conservation of matter?
All chemical reactions illustrate conservation of matter (because of their low energy transfer involving only electrons), while all nuclear reactions should uphold the conservation of energy (not matter, because matter is either created or destroyed in nuclear reactions).
Why is mass not conserved in chemical reactions?
This means that the total mass and energy before a reaction in a closed system equals the total mass and energy after the reaction. According to Einstein's famous equation, E = mc^2, mass can be transformed into energy and energy can be transformed into mass. This is not some exotic process, but in fact happens every time there is a reaction. Mass is therefore never conserved because a little of it turns into energy (or a little energy turns into mass) in every reaction. But mass+energy is always conserved. Energy cannot be created out of nothing. It can only be created by destroying the appropriate amount of mass according to E = mc^2.
How many conservation laws are there in classical mechanics?
There is two separate Conservation laws in classical mechanics, Conservation law of mass and Conservation of energy.
Who said there is no rise or drop in the weight of matter during a chemical reaction?
In 1785,the French scintist Antoine Lavoisier inferred from his research that 'there is no rise or drop in the weight of the matter during a chemical reaction'.In a chemical reaction the total weight of reactants is same as the total weight of the products formed due to the chemical reactions and this is called the law of conservation of matter.
Is mass an alternative form of energy?
Between mass and energy, energy is the more fundamental property. In fact, modern physicists just consider mass an alternate form of energy. For this reason, they don't usually call it the "Law of Conservation of Mass/Energy" but rather call it the "Law of Conservation of Energy" with the implication that this statement includes mass.
Should you tell the law of conservation of mass energy?
Actually you should tell it law of conservation of mass+energy.
What is the law of conservation of matter?
The law of conservation of matter is a fundamental principle of classical physics that states that matter cannot be created nor destroyed in any isolated system, but can only be converted from one form to another.
Is the law of conservation of matter the same as the law of conservation of energy?
The law of conservation of matter is virtually the same as the law of conservation of energy, which suggests that energy is never destroyed, but only changes form. An example of the law of conservation of matter can be observed when grapes ferment into wine.
What is the law of conservation of matter?
The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves.
Why was the conservation of matter important in the case of burned wood?
Once understood, the conservation of matter was of crucial importance in the progress from alchemy to the modern natural science of chemistry.
What is the energy of a beta particle?
About 89.28% of the time (10.72% is by electron capture), it decays to calcium-40 ( 40 Ca) with emission of a beta particle (β −, an electron) with a maximum energy of 1.33 MeV and an antineutrino, which is an antiparticle to the neutrino.
What is the conservation principle of mass energy?
Generally, in both chemical and nuclear reactions, some conversion between rest mass and energy occurs, so that the products generally have smaller or greater mass than the reactants. Therefore the new conservation principle is the conservation of mass-energy.
What is the formula for mass and energy?
One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula E = mc 2. In words, energy equals mass multiplied by the speed of light squared. Because the speed of light is a very large number, the formula implies that any small amount of matter contains a very large amount of energy. The mass of an object was seen to be equivalent to energy, to be interconvertible with energy, and to increase significantly at exceedingly high speeds near that of light. The total energy of an object was understood to comprise its rest mass as well as its increase of mass caused by increase in kinetic energy.
Can mass be created or destroyed?
The mass can neither be created nor destroyed.
Who discovered the principle of mass conservation?
Historically, already the ancient Greeks proposed the idea that the total amount of matter in the universe is constant. The principle of conservation of mass was first outlined by Mikhail Lomonosov in 1748. However, the law of conservation of matter (or the principle of mass/matter conservation) as a fundamental principle of physics was discovered in by Antoine Lavoisier in the late 18th century. It was of great importance in progressing from alchemy to modern chemistry. Before this discovery, there were questions like:
Mass Energy Relation
The law of conservation of mass is usually used to describe chemical changes.
1. Burning wood
In campsites, it’s popular for people to start fires using wood for cooking or warming themselves.
4. Disinfectant decomposition reactions
The disinfectant that you keep in your medicine cabinet for treating emergency minor cuts is most likely a bottle of hydrogen peroxide.
5. Synthesis reactions
Complex chemical substances (called compounds) can be formed in synthesis reactions.
6. Lit Candles
Have you wondered where your candle goes when it grows smaller or is consumed?
7. The vinegar and baking soda experiment
A common experiment which is done by students to prove the conservation of mass is to react vinegar with baking soda.
8. Algebraic equations
Algebraic equations can help us visualize the law of mass conservation. For example, the following is an algebraic equation;

The Law of Conservation of Matter in Special Relativity Theory
The Law of Conservation of Matter in Fluid Dynamics
- This principle is generally known as the conservation of matter principle. It states that the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves. This principle can be used in the analysis of flowing fluids. Conservation of mass in fluid dynamics states that all mass flow rates into a control volume are equal to all mas…
Continuity Equation
- The continuity equation is simply a mathematical expression of the principle of conservation of mass. For a control volume with a single inlet and a single outlet, the principle of conservation of mass states that, for steady-state flow, the mass flow rate into the volume must equal the mass flow rate out. ṁin = ṁout Mass entering per unit time = mass leaving per unit time This equatio…