Why is H2O a polar bond?
Why is water a polar molecule?
- The polarity of any molecule depends on these following factors:
- Its shape
- the difference of electronegativities between the atom and
- the net dipole moment in the molecule.
Why does H2O have polar covalent bonds?
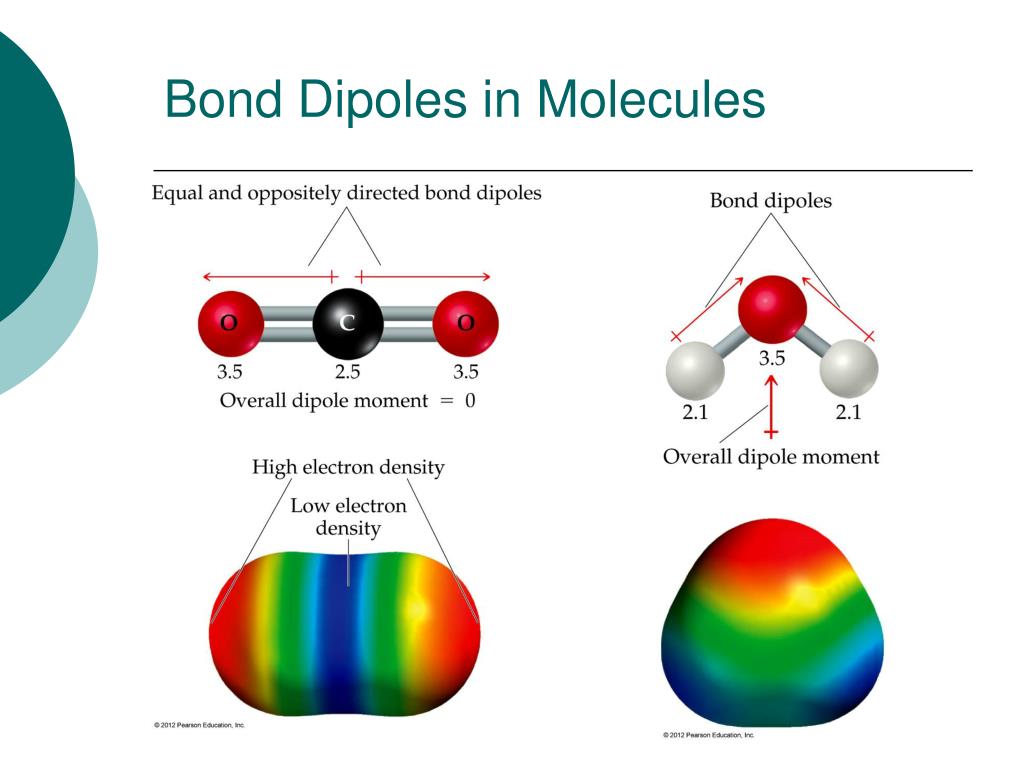
Water ( H 2 O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding. When solutes are added to water, they may be affected by ...
Does H2O represent a chemical bond?
Water (H 2 O) is a simple triatomic bent molecule with C 2v molecular symmetry and bond angle of 104.5° between the central oxygen atom and the hydrogen atoms. Despite being one of the simplest triatomic molecules, its chemical bonding scheme is nonetheless complex as many of its bonding properties such as bond angle, ionization energy, and electronic state energy cannot be explained by one ...
What type of bonding is present on H2O?
For H2O (water) the type of bonds between atoms are considered covalent (molecular). This occurs when two non-metal atoms bond and electron pairs are shared...
What determines the polarity of a molecule?
The polarity of any molecule depends on these following factors: Its shape. the difference of electronegativities between the atom and. the net dipole moment in the molecule. Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen ...
Why do both hydrogen atoms take up a position like the one shown in the figure?
Both the Hydrogen atoms take up a position like the one shown in the figure to keep the repulsive forces at the minimum. Due to this arrangement of both Hydrogen atoms the shape of the molecule is bent making the geometry of this molecule nonlinear.
What direction does the dipole moment go?
As a result of this pull, the dipole moment’s direction will be from Hydrogen towards the Oxygen atom on both sides . As a result of the dipole moment on both sides, there is a net dipole moment in this molecule. This would have been avoided if the shape of the molecule was linear.
What is the difference between oxygen and hydrogen?
Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen and Hydrogen’s electronegativities, it is much higher than 0.5, making the O-H bond a polar covalent bond.
Why is water a unique molecule?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
Why are the charges near the oxygen atom slightly negative?
This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
How many protons does water have?
Although there are poles in the molecules and a net dipole moment, it is an electrically neutral molecule. Each water molecule is made up of 10 protons, 10 electrons and a zero net charge.
What is the polarity of a molecule?
The polarity of a molecule majorly depends on its constituent atoms and their arrangement around the central atom. Polar molecules tend to attract water molecules, particularly through a Hydrogen bond.
How does polarity affect water?
The polarity of Water & Its Impacts on Physical Properties. The polarity of water shows many impacts on the physical properties of its molecules, primarily, the solvent properties. Initially, the water’s polarity clarifies its dissolvable properties.
What type of bond is a single, double, or triple bond?
Covalent bonds can be a single, double or triple bond on the basis of the number of electrons shared among the atoms. Covalent bonds can form polar or non-polar molecules. Polar bonds are formed when two molecules are created using a covalent bond.
Why are molecules polar?
Due to the formation of partial ionic charges, molecules become polar molecules with one side being charged highly positive and other side being highly negative. Molecules formed using an equal covalent bond to share electrons, with no ionic charge and symmetrical sharing of electrons are called nonpolar molecules.
How do ionic bonds form?
Ionic bonds are formed when atoms of opposite charge and signs attract each other to create neutralized molecules. Covalent bonds form in a condition where atoms can share electrons to create molecules. Covalent bonds can be a single, double or triple bond on the basis of the number of electrons shared among the atoms.
Why is water polar?
The polarity of water molecules shows many unique physical properties. One of the most particular reasons of water being a polar molecule is its bent shape. The bond angle between the O-H bonds in the H2O molecule is around 104.5 degrees.
What are the three states of matter that are standardized by the polarity and hydrogen bonds?
It is the only known compound that exists in all three states of matter, namely solid, liquid, and gaseous form even in the standard environment.
How to determine the polarity of a bond?
How do you determine the polarity of a bond?#N#Ans: The polarity of a bond can be determined by calculating the electronegativity difference between the participating atoms. For example, if the difference lies within 0.4 − 1.8, then the bond is a polar covalent bond.
How does polarity affect electrons?
As bond polarity involves the pulling of electrons towards itself, a high electronegative element will affect the extent of the polarity of bonds. The amount of the shifting of a bond pair of electrons will depend upon the relative electronegativity of the participating atoms.
What is Dipole and Dipole Moment?
A covalent bond involves the mutual sharing of valence electrons of the participating atom. This mutual sharing of electrons takes place to attain the stable electronic configuration of the neighbouring noble gas atom.
What type of bond is formed between hydrogen and chlorine?
Consequently, a polar covalent bond is formed between hydrogen and chlorine atom. This unequal sharing of the bonding pair of electrons results in a partial negative charge ( δ −) on the chlorine atom and a partial positive charge ( δ +) on the hydrogen atom.
Why do hydrogen bromide and hydrogen fluoride have non zero dipole moments?
Hydrogen bromide, hydrogen iodide, Hydrogen fluoride, Hydrogen Chloride have non-zero dipole moments that indicate the unsymmetrical charge distribution between two bonding atoms in the molecules. Due to the difference in electronegativity of the constituent atoms in heteronuclear diatomic molecules, the bond is always polar. Hence, the electron pair is not equally shared in hybridized disulfide orbital and shifted to the more electronegative atom.
What happens when atoms share electrons?
When atoms share their electrons, it tends to happen that the atom having higher electronegativity than the other pulls the shared pair of electrons more towards itself. This unequal sharing of electron pairs leads to the formation of dipoles ( 2 poles), and the molecules exhibiting this property are called Polar molecules.
Why is the dipole moment higher in H F than H I?
Hence Fluorine pulls the shared pair of electrons more towards itself. Thus, higher values of dipole moment indicate a higher degree of polarity .
