A coenzyme is an organic non-protein compound that binds with an enzyme to catalyze a reaction. Coenzymes
Cofactor
A cofactor is a non-protein chemical compound that is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations.
What is a coenzyme and why do they matter?
Ubiquinol is the active form of CoQ10 and plays a key role in producing the cellular energy your organs need to keep functioning - for example, cellular energy is what makes your heart pump. A lamp is powered by electricity. A car is powered by gas. Your heart and other organs are powered by cellular energy.
What is a coenzyme and what is its function?
The Functions of Coenzyme A
- Fatty Acid Synthesis. According to the "Molecular Biochemistry II" handbook, an online publication of the Rensselaer Polytechnic Institute, coenzyme A is the helper molecule that facilitates the oxidation pathway.
- Drug and Enzyme Functioning. Coenzyme A improves the functioning of some proteins, sugars and drugs, wrote Sareen S. ...
- Energy Production. ...
What describes the basic function of a coenzyme?
Related Biology Terms
- Catalyze – To cause or accelerate a reaction.
- Enzyme – A protein that catalyzes chemical reactions within an organism.
- Active Site – The region on an enzyme where substrates bind during a reaction.
- Substrate – The substance on which an enzyme acts to make a new product.
What is coenzyme and from what are they derived?
Types of Enzymes
- Coenzymes. These are reusable non-protein molecules that contain carbon (organic). ...
- Cofactors. Unlike coenzymes, true cofactors are reusable non-protein molecules that do not contain carbon (inorganic).
- Prosthetic groups. These can be organic vitamins, sugars, lipids, or inorganic metal ions. ...

What is a coenzyme made of?
Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types.
How is coenzyme produced?
During cell starvation, coenzyme A is synthesized and transports fatty acids in the cytosol to the mitochondria. Here, acetyl-CoA is generated for oxidation and energy production. In the citric acid cycle, coenzyme A works as an allosteric regulator in the stimulation of the enzyme pyruvate dehydrogenase.
What is a coenzyme simple definition?
Definition of coenzyme : a thermostable nonprotein compound that forms the active portion of an enzyme system after combination with an apoenzyme.
Where does CoA come from?
Acetyl-CoA is a metabolite derived from glucose, fatty acid, and amino acid catabolism. During glycolysis, glucose is broken down into two three-carbon molecules of pyruvate.
Which vitamins have a coenzyme form?
All of the water-soluble vitamins and two of the fat-soluble vitamins, A and K, function as cofactors or coenzymes.
What is coenzyme function?
A coenzyme is defined as an organic molecule that binds to the active sites of certain enzymes to assist in the catalysis of a reaction. More specifically, coenzymes can function as intermediate carriers of electrons during these reactions or be transferred between enzymes as functional groups.
What is coenzyme with example?
A coenzyme requires the presence of an enzyme in order to function. It is not active on its own. While enzymes are proteins, coenzymes are small, nonprotein molecules. Coenzymes hold an atom or group of atoms, allowing an enzyme to work. Examples of coenzymes include the B vitamins and S-adenosyl methionine.
What is difference between enzyme and coenzyme?
An enzyme is a protein that acts as a catalyst to increase the biochemical reaction rate without altering itself in the process, while a coenzyme is an organic non-protein molecule that is required by an enzyme to perform its catalytic activity.
What is the term used to describe an enzyme that is complete with its coenzymes and cofactors?
There are a few related terms also related to coenzymes: Apoenzyme is the name given to an inactive enzyme that lacks its coenzymes or cofactors. Holoenzyme is the term used to describe an enzyme that is complete with its coenzymes and cofactors. Holoprotein is the word used for a protein with a prosthetic group or cofactor.
What is a prosthetic group?
Prosthetic groups are enzyme partner molecules that bind tightly or covalently to the enzyme (remember, coenzymes bind loosely). While cosubstrates bind temporarily, prosthetic groups permanently bond with a protein.
What is the difference between coenzymes and enzymes?
They are intermediate carriers of an atom or group of atoms, allowing a reaction to occur. Coenzymes are not considered part of an enzyme's structure. They are sometimes referred to as cosubstrates . Coenzymes cannot function on their own and require the presence of an enzym e.
What are the three groups of chemicals that bind to enzymes?
Coenzymes, Cofactors, and Prosthetic Groups. Some texts consider all helper molecules that bind to an enzyme to be types of cofactors, while others divide the classes of chemicals into three groups: Coenzymes are nonprotein organic molecules that bind loosely to an enzyme. Many (not all) are vitamins or are derived from vitamins.
What are cofactors in biochemistry?
Some metallic elements have no nutritional value, but several trace elements function as cofactors in biochemical reactions, including iron, copper, zinc, magnesium, cobalt, and molybdenum.
What is an enzyme?
Anne Marie Helmenstine, Ph.D. Updated November 07, 2019. An enzyme is a macromolecule that catalyzes a chemical reaction. In other words, it makes an unfavorable reaction able to occur. Enzymes are built from smaller molecules to make an active subunit. One of the most important parts of an enzyme is the coenzyme.
Is AMP a cofactor?
Many (not all) are vitamins or are derived from vitamins. Many coenzymes contain adenosine monophosphate (AMP). Coenzymes may be described as either cosubstrates or prosthetic groups. Cofactors are inorganic species or at least nonprotein compounds that aid enzyme function by increasing the rate of catalysis.
What is the function of NAD + and NADP +?
In such enzymes, NAD + and NADP + do not dissociate with the release of the product, but stay tightly bound. Enzymes that catalyze reactions using a prosthetic group often have higher turnover rates than enzymes that allow the coenzyme to dissociate by virtue of eliminating slow association and dissociation steps. In addition, enzymes that bind and sequester a cofactor in the active redox state are rendered insensitive to regulation due to the redox state of the cell. This is due to the enzyme's ability to return the bound coenzyme to its original active redox state after catalysis so that it can perform subsequent reactions. Enzymes accomplish this in several ways. First, the enzyme can participate in alternating oxidation and reduction reactions with different substrates. Alternatively, the bound coenzyme can be reoxidized or reduced by a nonsubstrate molecule, such as ferrodoxin. In other reactions, the coenzyme can transiently oxidize or reduce a substrate, but return to the original redox state prior to product release. Finally, in some reactions, the coenzyme can accept only a pair of electrons and not the entire hydride ion. In this way, the bound coenzyme can promote catalysis without full oxidation or reduction occurring, eliminating the regeneration requirement. Enzymes that bind NAD + and NADP + as prosthetic groups are termed ‘nicotinoproteins’, which belong to a superfamily of metallo alcohol dehydrogenases.
What are the roles of uridine nucleotide coenzymes in carbohydrate metabolism?
UTP is involved in the synthesis of uridine nucleotide-sugars through the action of glucose-1-phosphate and galactose-1-phosphate uridyltransferases or UDP sugar pyrophosphorylases (EC 2.7.7.9 and EC 2.7.7.10). These enzymes have been found in mammal brain (Munch-Petersen et al., 1953; Breckenridge & Crawford, 1961; Bertoli & Segal, 1966 ). The role of UDP-sugars, may be of two kinds: on one hand, these coenzymes are implicated in glycosyl transfer; synthesis of hyaluronic acids, synthesis of glycolipids ( Burton et al., 1958; Kaufman et al., 1967 ), synthesis of psychosine ( Cleland & Kennedy, 1960) and of cerebrosides and sulphatides. On the other hand, the UDP-sugar reaction is involved in transformation of sugar structures. For example : the epimerization catalysed by UDP-glucose-4-epimerase (EC 5.1.3.2).
What is the role of nadh in the brain?
Its effects include the stimulation of dopamine, noradrenaline, and serotonin receptors, by which mechanism it is thought to increase mental alertness and clarity and improve concentration.
What is the most important food for the formation of red blood cells?
Folate is also essential for the formation and maturation of both red and white blood cells.28. Folate is widely distributed in many foods but found in the highest concentrations in liver, green leafy vegetables, dry beans, beets, oranges, cantaloupe, corn, sweet potatoes, wheat, and milk.
What is the role of folate in the metabolism of histidine?
It is required for the conversion of histidine to glutamic acid. In the presence of vitamin B 12, folate is necessary for the conversion of homocysteine to methione, therefore reducing plasma homocysteine, which is toxic to cells.
What is the function of folate?
Folate is a group of related compounds that function as enzyme co-substrates in many metabolic reactions of AAs and nucleotides. Dietary folate is absorbed into intestinal mucosal cells and reduced into its metabolically active form, tetrahydrofolic acid (FH4). FH4 functions as a single-carbon acceptor or donor necessary for DNA synthesis and is especially important in early fetal development. It is required for the conversion of histidine to glutamic acid. In the presence of vitamin B 12, folate is necessary for the conversion of homocysteine to methione, therefore reducing plasma homocysteine, which is toxic to cells. Folate is also essential for the formation and maturation of both red and white blood cells. 28
How much folate should I take for my baby?
The RDA of folate for infants is 65 to 80 μg/day and 150 to 400 μg for children. For adults, 300 to 400 μg/day is recommended; however, for women of childbearing age who might become pregnant, 400 to 600 μg is encouraged to prevent neural tube defects (NTDs).
Which vitamin is a coenzyme?
Vitamins with possible coenzyme functions are B 12 which is associated with tetrahydrofolate in some of its functions, and vitamin A , which is said to function, in the form of its aldehyde retinene, as a cofactor in the visual cycle. View chapter Purchase book.
What is the coenzyme of folate?
A coenzyme of folate is tetrahydrofolate (THF), a carrier of one-carbon units, such as methyl groups (—CH 3 ). One-carbon units arise primarily from the metabolism of amino acids. They are needed to interconvert amino acids and to synthesize purines and pyrimidines for the formation of RNA and DNA.
What is the function of biocytin?
Biotin as the coenzyme biocytin functions in carboxylation reactions that convert odd-carbon-numbered amino acids and fatty acids to even-carbon-numbered compounds, which can then be metabolized. Biocytin is also necessary for the synthesis of pyrimidines and the formation of urea.
What are the cofactors of vitamins?
All of the water-soluble vitamins and two of the fat-soluble vitamins, A and K , function as cofactors or coenzymes. Coenzymes participate in numerous biochemical reactions involving energy release or catabolism, as well as the accompanying anabolic reactions (Figure 1 ). In addition, vitamin cofactors are critical for processes involved in proper vision, blood coagulation, hormone production, and the integrity of collagen, a protein found in bones. ( See RETINOL | Physiology .)
What is the aldehyde form of vitamin A?
The aldehyde form of vitamin A, retinal, is a cofactor for apoproteins in the eye called opsins. Opsins are responsible for dim-light vision in the rods (rhodopsin) and are involved in color and bright-light vision in the cone of the retina (iodopsin).
Which hormone regulates the synthesis of flavokinase?
In the kidney, the synthesis of flavokinase and hence of flavin coenzymes is controlled by aldosterone.
What is the source of phosphopantetheine?
Coenzyme A is also the source of the phosphopantetheine group that is added as a prosthetic group to proteins such as acyl carrier protein and formyltetrahydrofolate dehydrogenase. Some of the sources that CoA comes from and uses in the cell.
What is the CoA pathway?
This pathway is regulated by product inhibition. CoA is a competitive inhibitor for Pantothenate Kinase, which normally binds ATP. Coenzyme A, three ADP, one monophosphate, and one diphosphate are harvested from biosynthesis.
What is a coenzyme that is not attached to an acyl group?
When it is not attached to an acyl group, it is usually referred to as 'CoASH' or 'HSCoA'. This process facilitates the production of fatty acids in cells, which are essential in cell membrane structure.
How is coenzyme A synthesized?
Coenzyme A can be synthesized through alternate routes when intracellular coenzyme A level are reduced and the de novo pathway is impaired. In these pathways, coenzyme A needs to be provided from an external source, such as food, in order to produce 4′-phosphopantetheine.
What does the name coenzyme A stand for?
The coenzyme was named coenzyme A to stand for "activation of acetate ". In 1953, Fritz Lipmann won the Nobel Prize in Physiology or Medicine "for his discovery of co-enzyme A and its importance for intermediary metabolism".
What is the role of coenzyme A in fatty acid synthesis?
Fatty acid synthesis. Since coenzyme A is, in chemical terms, a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. It assists in transferring fatty acids from the cytoplasm to mitochondria.
What is the name of the molecule that degrades coenzyme A?
Ectonucleotide pyrophosphates ( ENPP) degrade coenzyme A to 4′-phosphopantetheine, a stable molecule in organisms. Acyl carrier proteins (ACP) (such as ACP synthase and ACP degradation) are also used to produce 4′-phosphopantetheine.
What enzymes use coenzyme A?
All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it, or a thioester form of it, as a substrate. It is used as a supplement for the hypothetical treatment of acne.
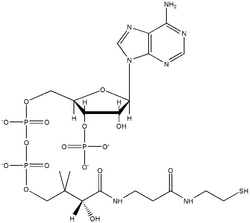
What is the coenzyme A?
Coenzyme A is a coenzyme containing pantothenic acid, adenosine 3-phosphate 5-pyrophosphate, and cysteamine; involved in the transfer of acyl groups, notably in transacetylations. Coenzyme A is a thiol comprising a panthothenate unit in phosphoric anhydride linkage with a 3',5'-adenosine diphosphate unit; and an aminoethanethiol unit.
What is the name of the coenzyme that is responsible for the synthesis and oxidation of fatty
It derives from an ADP. It is a conjugate acid of a coenzyme A (4-). Coenzyme A ( CoA , CoASH , or HSCoA) is a coenzyme, well known for it's role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, ...
Is HMDB a free resource?
LICENSE. HMDB is offered to the public as a freely available resource . Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page).
What is the purpose of CoQ10?
Overview. Coenzyme Q10 (CoQ10) is an antioxidant that your body produces naturally. Your cells use CoQ10 for growth and maintenance. Levels of CoQ10 in your body decrease as you age.
What is CoQ10 supplement?
CoQ10 dietary supplements are available as capsules, chewable tablets, liquid syrups, wafers and by IV. CoQ10 might help prevent or treat certain heart conditions, as well as migraine headaches.
Does CoQ10 help with Parkinson's?
Recent research suggests that even high doses of CoQ10 don't seem to improve symptoms in people with Parkinson's disease. Statin-induced myopathy. Some research suggests that CoQ10 might help ease the muscle weakness and pain sometimes associated with taking statins. Migraines.
Does CoQ10 help with heart failure?
Heart conditions. CoQ10 has been shown to improve symptoms of congestive heart failure. Although findings are mixed, CoQ10 might help reduce blood pressure. Some research also suggests that when combined with other nutrients, CoQ10 might aid recovery in people who've had bypass and heart valve surgeries.
How long before surgery can you stop taking Coenzyme Q10?
Your blood pressure may need to be checked while you are taking Coenzyme Q10. If you need surgery, stop taking Coenzyme Q10 at least 2 weeks ahead of time. Store at room temperature, away from light, heat, and moisture. Keep the medicine bottle closed when not in use. Detailed Coenzyme Q10 dosage information.
Is Coenzyme Q10 safe for pregnant women?
Coenzyme Q10 is considered possibly safe to use during pregnancy. However, do not use this product without medical advice if you are pregnant . It is not known whether ubiquinone passes into breast milk or if it could harm a nursing baby. Do not use this product without medical advice if you are breast-feeding a baby.
Is Coenzyme Q10 effective?
However, research has shown that this medicine may not be effective in treating these conditions. Research also has shown that Coenzyme Q10 is not likely to be effective in increasing athletic performance. Other uses not proven with research have included treating asthma, COPD, cancer, diabetes, certain heart problems, fibromyalgia, hepatitis C, ...
Is Coenzyme Q10 a vitamin?
Coenzyme Q10 is a vitamin-like substance that is made naturally in the body. This medicine is also known as Coenzima, Ubidcarenone, Ubidécarénone, and Ubiquinol. Coenzyme Q10 is likely effective in alternative medicine as an aid in treating coenzyme Q-10 deficiency, or reducing the symptoms of mitochondrial disorders ...
Is Q10 a regulated product?
Coenzyme Q10 is often sold as an herbal supplement. There are no regulated manufacturing standards in place for many herbal compounds and some marketed supplements have been found to be contaminated with toxic metals or other drugs.
Can you use Coenzyme Q10?
Use Coenzyme Q10 exactly as directed on the label, or as directed by your doctor, pharmacist, or other healthcare provider. Do not use more of this product than is recommended on the label. When considering the use of herbal supplements, seek the advice of your doctor.
Can you take Coenzyme Q10 without medical advice?
Do not take Coenzyme Q10 without medical advice if you are using any of the following medications: omega-3 fatty acids; vitamins (especially A, C, E, or K); blood pressure medicine; cancer medicine; or. warfarin ( Coumadin, Jantoven ). This list is not complete.
What is the role of tetrahydrofolic acid in the synthesis of amino acids?
A deficiency of this coenzyme produces anemia.
What is the role of B6 in the body?
Vitamin B 6 (pyridoxine) . Water-soluble coenzyme eliminated through urine, so it has to be replaced through the diet: wheat germ, cereals, eggs, fish and vegetables, among other foods. It intervenes in the metabolism of neurotransmitters and has a prominent role in the energy circuit. Lipoic acid .
What is BH4?
Also called sapropterin or BH 4 , it is an essential coenzyme for the synthesis of nitric oxide and the hydroxylases of aromatic amino acids. Its deficiency is linked to low neurotransmitters such as dopamine or serotonin. Coenzyme Q10 (ubiquinone) .
What is biocytin used for?
Biocytin . Indispensable in the transfer of carbon dioxide, it occurs naturally in blood serum and urine. It is used in scientific research as a dye for nerve cells. Vitamin B 2 (riboflavin) .
What is the role of vitamin K in the body?
Vitamin K . Linked to the blood coagulation factor, it acts as an activator of different plasma proteins and osteocalcina. It is achieved in three ways: Vitamin K 1 , abundant in any diet and of vegetable origin; Vitamin K 2 of bacterial origin and Vitamin K 3 of synthetic origin. Cofactor F420 .
What is GSH in the body?
Glutathione (GSH) . This tripeptide is an antioxidant and cellular protector of free radicals and other toxins. It is synthesized in the liver essentially, but any human cell is able to manufacture it from other amino acids, such as glycine.
What is the main molecule of energy transfer from one cell to the other?
Adenosine triphosphate (ATP) . This molecule is used by all living beings to feed their chemical reactions with energy and used in the synthesis of cellular RNA. It is the main molecule of energy transfer from one cell to the other. S-adenosyl methionine (SAM) .
Overview
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitami…
Discovery of structure
Coenzyme A was identified by Fritz Lipmann in 1946, who also later gave it its name. Its structure was determined during the early 1950s at the Lister Institute, London, together by Lipmann and other workers at Harvard Medical School and Massachusetts General Hospital. Lipmann initially intended to study acetyl transfer in animals, and from these experiments he noticed a unique factor tha…
Biosynthesis
Coenzyme A is naturally synthesized from pantothenate (vitamin B5), which is found in food such as meat, vegetables, cereal grains, legumes, eggs, and milk. In humans and most living organisms, pantothenate is an essential vitamin that has a variety of functions. In some plants and bacteria, including Escherichia coli, pantothenate can be synthesised de novo and is therefore not considered es…
Function
Since coenzyme A is, in chemical terms, a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. It assists in transferring fatty acids from the cytoplasm to mitochondria. A molecule of coenzyme A carrying an acyl group is also referred to as acyl-CoA. When it is not attached to an acyl group, it is usually referred to as 'CoASH' or 'HSCoA'. This p…
Use in biological research
Coenzyme A is available from various chemical suppliers as the free acid and lithium or sodium salts. The free acid of coenzyme A is detectably unstable, with around 5% degradation observed after 6 months when stored at −20 °C, and near complete degradation after 1 month at 37 °C. The lithium and sodium salts of CoA are more stable, with negligible degradation noted over several months at various temperatures. Aqueous solutions of coenzyme A are unstable above pH 8, wit…
Non-exhaustive list of coenzyme A-activated acyl groups
• Acetyl-CoA
• fatty acyl-CoA (activated form of all fatty acids; only the CoA esters are substrates for important reactions such as mono-, di-, and triacylglycerol synthesis, carnitine palmitoyl transferase, and cholesterol esterification)
• Acetoacetyl-CoA
Bibliography
• Nelson, David L.; Cox, Michael M. (2005). Lehninger: Principles of Biochemistry (4th ed.). New York: W .H. Freeman. ISBN 978-0-7167-4339-2.