Why are atomic spectra continuous?
Quick answer: Atomic spectra are continuous because the energy levels of electrons in atoms are quantized. The electrons in an atom can have only certain energy levels. There is no middle ground. If an electron is excited to a new energy level, it jumps to that level instantaneously.
What does atomic emission spectra represent?
This release occurs in the form of light of a specific wavelength (colour). Hence, atomic emission spectra represent the electrons returning to lower energy levels.
What is a Continuous Spectrum?
In general terms, a spectrum is the set of values between two extremes. The continuous spectrum definition is all of the values in the spectrum without any gaps, skips, or breaks. The spectrum of visible light can be seen when white light is shined through a prism.
The Spectrum of an Electromagnetic Wave
Visible light is a form of electromagnetic radiation but, in actuality, it is only a small section of the larger electromagnetic spectrum. Electromagnetic radiation travels in the form of a wave, in a smooth, up-and-down cyclic motion.
Types of Spectrums of EM Waves from the Sun
The sun shines white light and, in fact, regular sunlight that is aimed through a prism will disperse into the rainbow of visible colors. However, if white light from the sun is analyzed using a spectrometer (a device that measures wavelengths of light), it is clear that there are gaps in the spectrum.
Emitted vs Absorbed Light
The following questions encourage students to think about which process causes us to see what things.
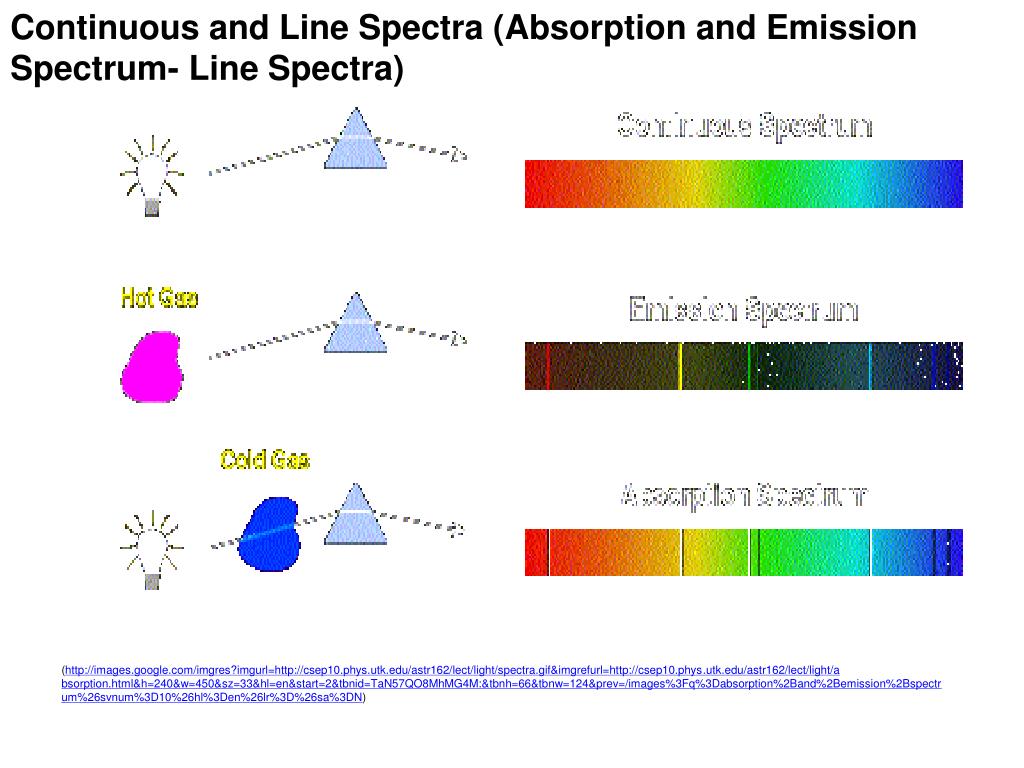
What is continuous spectrum?
What is a Continuous Spectrum? The term continuous spectrum is mostly found in physics and mainly involves light and colors found therein. Spectra also help us understand how atoms absorb different light energies to provide the color we see. There are two main types of spectra.
When is continuous spectrum possible?
It is also possible to obtain continuous spectra when you heat up different materials until they glow. The correct continuous spectrum definition therefore exhibits when, within a given limit, all the wavelengths are present.
What is the spectrum of a rainbow?
If the rainbow that is produced has all the colors from red to violet, and has no gaps between them, then it is referred to as a continuous spectrum . A beam of pure white light attainable is some laboratories often contain this spectrum.
Why do incandescent bulbs produce continuous spectrum?
Only incandescent light bulbs produce continuous spectrums. This is because the light source is a heated metal wire.
Why do atoms emit white light at the glowing point?
This is because atoms emit white light at glowing point and have thus given away all the energy absorbed. In order to generate complete continuous spectra, we need to put together absorption and emission spectra.
Where can you see the spectrum?
Apart from a rainbow, you can sometimes see the continuous spectrum around the sun or moon.
Can you have both continuous and line spectra?
Continuous and line spectra and while these are generally different, it is possible to have both of them . Continuous spectrum can be learnt vividly in the context of light and electromagnetic spectrum. Here is a brief definition and description of these spectra as well as their differences: