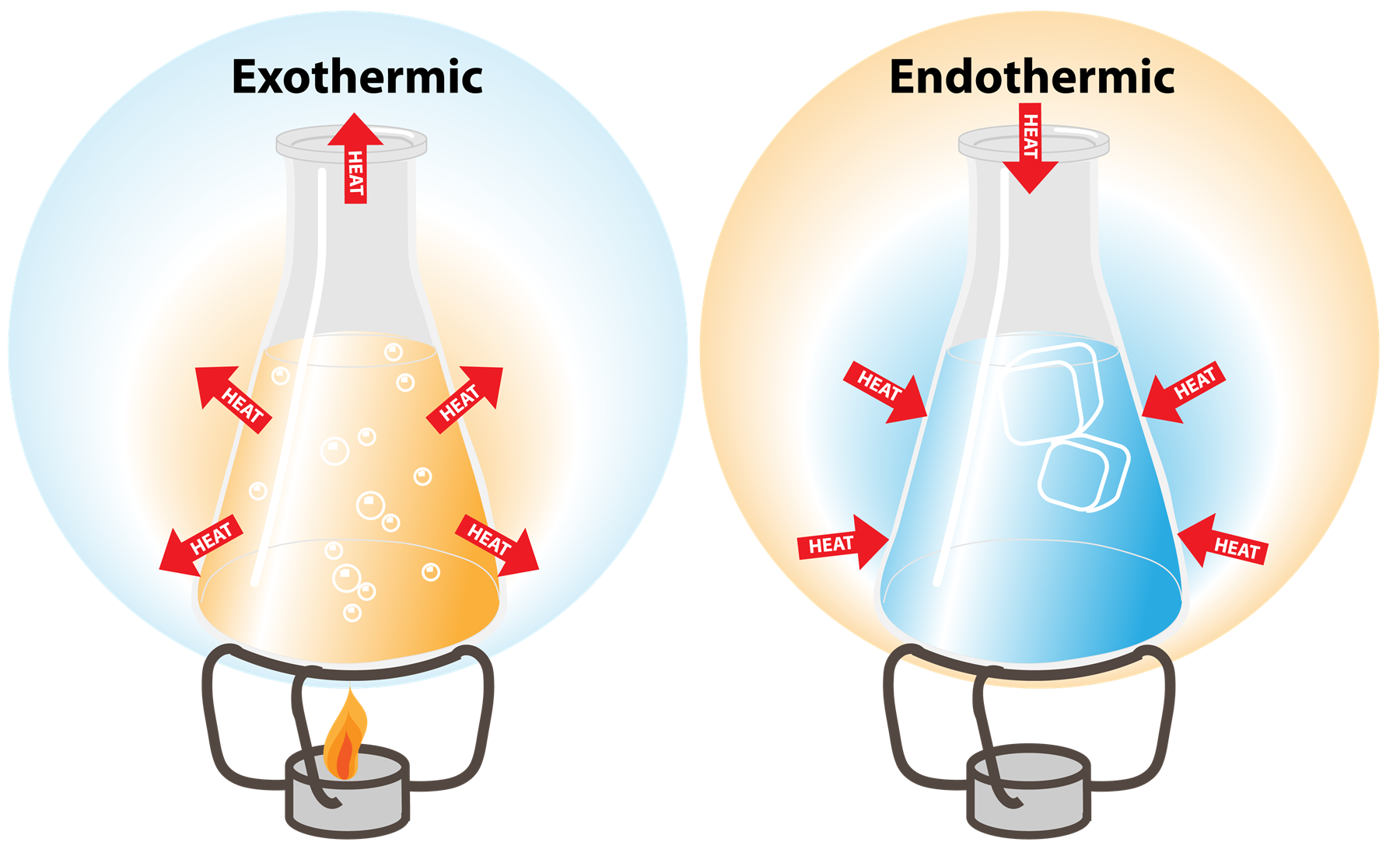
What is an example of an endothermic?
The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, dry ice, alkanes cracking, thermal decomposition, ammonium chloride in water and much more. Exothermic Reactions
What are some examples of endothermic reactions?
Some other Examples of Endothermic Reaction in Everyday Life:
- Salt Dissolving in Water
- Cracking of Alkalines
- Disappearance of Naphthalene Balls
- Thermal Decomposition
- Pressure Cookers
- Hydrolysis
- Melting of Ice, etc.
What are the everyday life examples of endothermic reactions?
What are the everyday life examples of endothermic reactions?
- Photosynthesis.
- Melting ice.
- Evaporating liquid water.
- Sublimation of carbon dioxide (dry ice)
- Cracking of alkanes.
- Thermal decomposition reactions.
- Electrolytic decomposition of sodium chloride into sodium hydroxide and hydrogen chloride.
- Dissolving ammonium chloride in water.
What are some examples of endothermic animals?
endothermic animals. Also called “warm-blooded” are animals that can regulate their temperature regardless of the environmental temperature. For example: dog, canary, human. Heliothermy. It is the capture of heat offered by solar radiation. It is a common method in reptiles and also used even by endodermal animals.
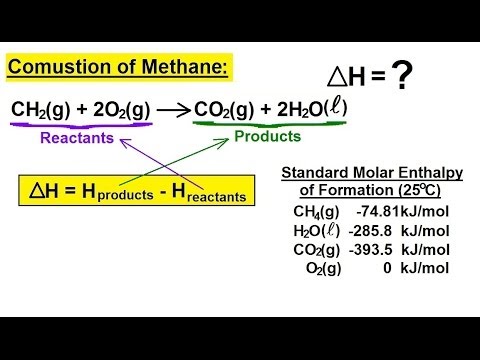
What is an example of an exothermic?
2) Rain: Condensation of water vapor into rain releasing energy in the form of heat is an example of an exothermic process.
What is endothermic and exothermic example?
The chemical reactions that release heat energy are called exothermic reactions. Example: C(g)+O2(g)→CO2(g)+Heat Energy. The chemical reactions in which heat energy is absorbed are called endothermic reactions. Example: CaCO3Heat→CaO+CO2.
What are 5 examples of an endothermic reaction?
A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, dry ice, alkane cracking, thermal decomposition, ammonium chloride in water and much more.
What are 5 examples of exothermic reactions?
Here are some of the examples of exothermic reactions:Making of an Ice Cube. Making an ice cube is a process of liquid changing its state to solid. ... Snow Formation in Clouds. ... Burning of a Candle. ... Rusting of Iron. ... Burning of Sugar. ... Formation of Ion Pairs. ... Reaction of Strong Acid and Water. ... Water and Calcium Chloride.More items...
What is an Endothermic Reaction?
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Physical processes can be endothermic as well – Ice cubes absorb heat energy from their surroundings and melt to form liquid water (no chemical bonds are broken or formed).
What is the difference between endothermic and exothermic reactions?
As the names suggest, the primary difference between endothermic and exothermic reactions is that the former absorbs heat from the surroundings whereas the latter involves a release of heat. Some other differences between these types of chemical reactions are tabulated below. Endothermic Reaction. Exothermic Reaction.
Is sweating an exothermic reaction?
However, sweating is not an exothermic reaction. Chemical reactions can involve the breaking of existing chemical bonds, the formation of new bonds, or both. The evaporation of sweat does not involve any chemical changes but it does involve a change in physical phase (from liquid to vapour). Therefore, evaporation is said to be a physical ...
Is nitric oxide endothermic or endothermic?
The formation of nitric oxide from the reaction between nitrogen and oxygen is endothermic since it involves the absorption of approximately 180.5 kilojoules of heat for every mole of N 2 and O 2.
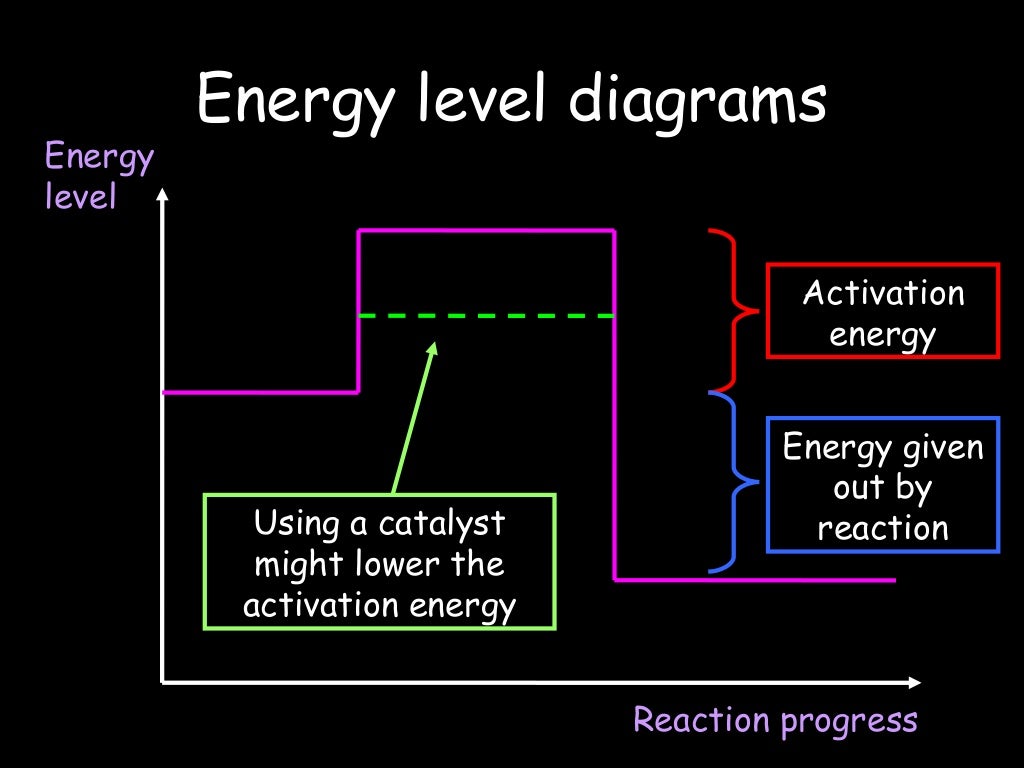
Is the potential energy of the product higher than that of the reactant?
For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. On the other hand, the potential energy of the product in an endothermic reaction is higher than that of the reactants. This gap in the potential energy accounts for the energy that was absorbed by the system during the chemical reaction.
Is evaporation an endothermic process?
Therefore, evaporation is said to be a physical endothermic process rather than an endothermic reaction. Any process that absorbs heat from its surroundings is an endothermic process. Therefore, all endothermic reactions are endothermic processes. However, the opposite is not true. Many endothermic processes involve physical changes rather ...
What is endothermic reaction?
Endothermic Reaction Defined. Chemical reactions are all about the energy. In an endothermic reaction, heat is used for the reaction to occur. The heat energy breaks the bonds in the substance causing the reaction. As the heat is absorbed, the product will be colder.
What is the difference between endothermic and exothermic?
However, the two are different. The main difference is endothermic absorbs heat while exothermic produces heat.
How to make a drink colder?
A fun endothermic process you use every day is getting your drink nice and cold by adding ice. The ice pulls the heat from the water to melt the ice making your drink nice and cold.
Is bread an endothermic product?
Nothing is better than fresh-baked bread. In addition to smelling delicious, baking bread is an endothermic example. The flour, yeast, and other ingredients used in creating the dough are heated. They absorb the heat making chemical reactions happen. And, the product is quite tasty!
Is chemical fire exothermic or exothermic?
A chemical fire is a great exothermic example. The right, or should you say the wrong, combination can make the chemicals burst into flames. Flames give off heat. Therefore, in an exothermic reaction, heat is released and temperatures go up rather than going down.
Is cooking an egg endothermic?
You might not have realized cooking an egg was an endothermic reaction. However, energy from the pan is absorbed to cook the egg in this endothermic reaction. Try it at home! It’s a yummy example.
Do endothermic reactions cause cold?
Well, some reactions cause cold. Endothermic reactions absorb heat and lead to the product being colder. Dive into some fun examples of endothermic reactions at play. Find out how they are different from exothermic reactions through examples. endothermic reaction examples.
What are some examples of endothermic reactions?
Examples of Endothermic: 1. A "cold pack" used in sports gets cold when the chemical inside mixes with the water bag and new bonds form causing a decrease in temperature. 2. Photosynthesis involves the input of the sun's energy into a chemical reaction to make food for plants. 3.
What does endothermic mean?
So this term endothermic means heat energy is taken in and used in the making of the new bonds. Many processes can also be endothermic without being a chemical reaction. This means that heat energy is input into the system to cause a physical change.
What are some examples of endotherms?
In contrast, ectotherms depend on external sources to generate needed body heat. Common examples of endotherms are what we call warm-blooded animals, such as mammals. As humans, we are also endotherms!
Is a human an endotherm?
Humans and most warm-blooded animals are endotherms, however, there are some exceptions on this list! Read on to learn more about 15 examples of endotherms.
Do endotherms have a temperature?
The body temperature of endotherms doesn’t fluctuate with external weather conditions. Meaning, they have the ability to keep themselves warm through metabolic processes. Endotherms can burn energy cells to produce heat and are often referred to as warm-blooded as a result.
Do endotherms need more food?
The metabolic rate of endotherms will increase when external temperatures are high, so they typically require more food during warmer weather. They also have different methods to prevent overheating. Generally, endotherms have more stamina than ectotherms that need to absorb heat from the sun. Additionally, endotherms can be active both during the day and night.