What is face centered unit cell?
Face-centred Cubic Unit Cell (FCC) An FCC unit cell contains atoms at all the corners of the crystal lattice and at the centre of all the faces of the cube. The atom present at the face-centered is shared between 2 adjacent unit cells and only 1/2 of each atom belongs to an individual cell.
What is fcc (face centered cubic)?
Face-centered cubic (FCC or cF) is the name given to a type of atom arrangement found in nature. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face. The atoms at the corner of the cube are ...
What is face-centered cubic (CCP)?
Face-centered cubic (also known as FCC, cF, cubic close-packed or CCP) is the name given to a type of atom arrangement found in nature. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face.
What is FCC unit cell?
An FCC unit cell contains atoms at all the corners of the crystal lattice and at the centre of all the faces of the cube. The atom present at the face-centered is shared between 2 adjacent unit cells and only 1/2 of each atom belongs to an individual cell. In FCC unit cell atoms are present in all the corners of the crystal lattice
What is a face centered cubic lattice?
How much packing does a face centered cubic crystal have?
How many atoms are in a FCC cell?
What is the maximum APF of a FCC crystal?
What is the APF of a sphere?
What is the FCC structure?
How many NNs are there in a cubic crystal?
See 2 more
What is face Centre cubic unit cell?
An FCC unit cell contains atoms at all the corners of the crystal lattice and at the centre of all the faces of the cube. The atom present at the face-centered is shared between 2 adjacent unit cells and only 1/2 of each atom belongs to an individual cell.
What is face-centered cell?
Definition of face-centered : relating to or being a crystal space lattice in which each cubic unit cell has an atom at the center and at the corners of each face — compare body-centered.
What is FCC and BCC?
BCC – Body Centred Cubic – and FCC – Face Centred Cubic – are descriptions of the arrangement of atoms in crystal structures. Most metal and alloys are crystalline, which means that their atoms arrange themselves in an ordered pattern.
What is FCC give any two examples?
physical metallurgy. …of each face (known as face-centred cubic, or fcc). Examples of metals with the hcp type of structure are magnesium, cadmium, zinc, and alpha titanium. Metals with the fcc structure include aluminum, copper, nickel, gamma iron, gold, and silver.
How many atoms are in FCC unit cell?
Face Centered Cubic This unit cell uses 14 atoms, eight of which are corner atoms (forming the cube) with the other six in the center of each of the faces.
How many FCC unit cells are there?
As the F.C.C unit cells consists of 6 faces so it is equally shared by 6 unit cells.
What is the difference between FCC and BCC crystal structure?
The BCC unit cell consists of a net total of two atoms, the one in the center and eight eighths from the corners. In the FCC arrangement, again there are eight atoms at corners of the unit cell and one atom centered in each of the faces. The atom in the face is shared with the adjacent cell.
Which is stronger BCC or FCC?
BCC metals are infact stronger than FCC metals. HCP metals are the most brittle.
What is BCC FCC and HCP?
The hexagonal closest packed (hcp) has a coordination number of 12 and contains 6 atoms per unit cell. The face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. The body-centered cubic (bcc) has a coordination number of 8 and contains 2 atoms per unit cell.
How do you draw an FCC structure?
0:0717:08The Face Centred Cubic Crystal Structure - YouTubeYouTubeStart of suggested clipEnd of suggested clipFirst at the corners. And then as the name implies in some other place in the center of the faces inMoreFirst at the corners. And then as the name implies in some other place in the center of the faces in this case and I'll show you that in a moment.
What is coordination number of FCC?
Coordination number – the number of nearest neighbor atoms or ions surrounding an atom or ion. For FCC and HCP systems, the coordination number is 12. For BCC it's 8.
What is bcc and FCC iron?
Iron has two different crystal structures at atmospheric pressure: the body centered cubic (bcc) and the face centered cubic (fcc). In the ground state the bcc α-phase is stable, and at the temperature T=1184 K (A3 point), α-Fe transforms into fcc α-Fe, which is stable up to 1665 K(A4 point).
How do you know if an element is bcc or FCC?
If the unit cell also contains an identical component in the center of the cube, then it is body-centered cubic (bcc) (part (b) in Figure 12.5). If there are components in the center of each face in addition to those at the corners of the cube, then the unit cell is face-centered cubic (fcc) (part (c) in Figure 12.5).
What are bcc metals?
The bcc unit cell has a packing factor of 0.68. Some of the materials that have a bcc structure include lithium, sodium, potassium, chromium, barium, vanadium, alpha-iron and tungsten. Metals which have a bcc structure are usually harder and less malleable than close-packed metals such as gold.
What is the difference between CCP and FCC?
The cubic closed packing is CCP, FCC is cubic structures entered for face. When we put the atoms in the octahedral void, the packing is of the form of ABCABC, so it is known as CCP, while the unit cell is FCC. The unit cell consists of four layers of atoms in a cubic close-packed (ccp) arrangement of atoms.
What Does Face-Centered Cubic (FCC) Mean?
Face-centered cubic (FCC or cF) is the name given to a type of atom arrangement found in nature. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face.
What is the APF of a crystal structure?
The packing density, also known as the atomic packing factor (APF), is essentially the fraction of the volume of atoms that occupy a crystal structure . The APF of an FCC structure is equal to the volume of the atoms in the unit cell divided by the volume of the unit cell. Therefore: APF FCC = V atoms /V unit cell.
What is the FCC packing arrangement?
In nature, several packing arrangements are possible, including the face-centered cubic arrangement. One of the defining features of FCC is that the atoms are packed as close together as theoretically possible. The atoms from one layer nest themselves snugly into the empty space of each adjacent layer.
Why are FCC atoms packed closer than other cell arrangements?
Due to their packing arrangement, FCC metals are typically softer and more ductile than their BCC counterparts are , which may be an important factor when selecting materials for a given application.
How many atoms are in a face centered cubic unit cell?
A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face. The atoms at the corner of the cube are shared with eight other unit cells.
How many atoms are in a unit cell?
Using this concept, the total number of atoms in the FCC unit cell structure is 4; six halves at each of the faces, plus eight one-eighth atoms at the corners.
What is the value of APF FCC?
Cancelling common terms, we get APF FCC = 0.74. In other words, 74% of the volume of the unit cell is occupied by atoms. This value is referred to as close-packed because it is not possible to pack the atoms any closer to achieve a higher APF.
What is a face centered cubic?
In a face-centered cubic (fcc) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the center of each of the faces of the cube.
How many atoms are in a unit cell?
In a face-centered cubic (fcc) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the center of each of the faces of the cube. In a fcc arrangement, a unit cell contains (8 corner atoms × ⅛) + (6 face atoms × ½) = 4 atoms.
What are the three basic cubic units?
We will focus on the three basic cubic unit cells: primitive cubic (from the previous section), body-centered cubic unit cell, and face-centered cubic unit cell —all of which are illustrated in Figure 1.
Where are atoms located in a face-centered solid?
Figure 3. A face-centered cubic solid has atoms at the corners and, as the name implies, at the centers of the faces of its unit cells.
How many atoms do you touch in a cubic structure?
Figure 2. In a body-centered cubic structure, atoms in a specific layer do not touch each other. Each atom touches four atoms in the layer above it and four atoms in the layer below it.
How many atoms are in a BCC cell?
A BCC unit cell contains two atoms: one-eighth of an atom at each of the eight corners (8 × 1 8 1 8 = 1 atom from the corners) plus one atom from the center. Any atom in this structure touches four atoms in the layer above it and four atoms in the layer below it. Thus, an atom in a BCC structure has a coordination number of eight.
What is the simplest repeating unit of a crystalline solid?
In the previous section, we identified that unit cells were the simplest repeating unit of a crystalline solid and examined the most basic unit cell, the primitive cubic unit cell. In this section, we continue by looking at two other unit cell types, the body-centered cubic and the face-centered cubic unit cells.
How many atoms are in each layer of CCP?
In CCP, there are three repeating layers of hexagonally arranged atoms. Each atom contacts six atoms in its own layer, three in the layer above, and three in the layer below. In this arrangement, each atom touches 12 near neighbors, and therefore has a coordination number of 12.
What is unit cell geometry?
The unit cell is defined as the smallest repeated unit with full crystal structure symmetry. The unit cell geometry is known as a parallelepiped, providing six lattice parameters taken as the lengths of the edges of the cells (a, b , c) and the angles between them (α, β, ).
How many units are in a primitive cubic unit cell?
Primitive Cubic Unit Cell. In the primitive cubic unit cell, the atoms are present only at the corners. Every atom at the corner is shared among 8 adjacent unit cells. There are 4 unit cells in the same layer and 4 in the upper (or lower) layer. Therefore, a particular unit cell has the only 1/8 th of an atom.
What is a Unit Cell?
The smallest repeating unit of the crystal lattice is the unit cell, the building block of a crystal.
Where are atoms in a BCC cell?
A BCC unit cell has atoms at each corner of the cube and an atom at the centre of the structure. The diagram shown below is an open structure. According to this structure, the atom at the body centre wholly belongs to the unit cell in which it is present. In BCC unit cell every corner has atoms.
Where are the atoms in a primitive cubic unit cell found?
The atoms in the primitive cubic unit cell are present only at the corners
How many atoms are in a unit cell?
Therefore, the total number of atoms in a unit cell = 4 atoms.
Which cell is the atom at the body centres?
According to this structure atom at the body centres wholly belongs to the unit cell in which it is present.
What is a face centered cubic lattice?
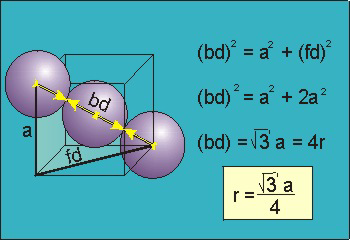
The face-centered cubic lattice is a cube with an atom on each corner and each face. Using the hard sphere model, which imagines each atom as a discrete sphere, the FCC crystal has each atom touch along the face diagonal of the cube. That means that the face diagonal has a length of , so with a bit of geometry we find that the lattice parameter , ...
How much packing does a face centered cubic crystal have?
As you can see, face-centered cubic crystals have 74% packing.
How many atoms are in a FCC cell?
It is one of the most common structures for metals. FCC has 4 atoms per unit cell, lattice constant a = 2R√2, Coordination Number CN = 12, and Atomic Packing Factor APF = 74%. FCC is a close-packed structure with ABC-ABC stacking.
What is the maximum APF of a FCC crystal?
FCC is close-packed, which means it has the maximum APF of 0.74. Because FCC has 12 independent slip systems, it is also very ductile.
What is the APF of a sphere?
Since we use the hard sphere model, each point inside the cell is either part of an atom, or part of the void. APF is basically the fraction of atoms to void.
What is the FCC structure?
FCC is one of the most stable crystal structures and has the highest packing density. In some textbooks, FCC may also be abbreviated as CCP, which stands for Cubic Close-Packed. The face-centered cubic cell belongs to space group #225 or , Strukturbericht A 1, and Pearson symbol cF4 . Cu is the prototype for FCC.
How many NNs are there in a cubic crystal?
In a face-centered cubic crystal, each atom has 12 nearest neighbors (NN). That’s the theoretical maximum number of NNs possible–each of those NNs contributes a bond, giving the crystal structure very high stability.

Body-Centered Cubic Cells
Face-Centered Cubic Cells
- Many other metals, such as aluminum, copper, and lead, crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and at the centers of each face, as illustrated in Figure 3. This arrangement is called a face-centered cubic (FCC) solid. A FCC unit cell contains four atoms: one-eighth of an atom at each of the eight co...
Key Concepts and Summary
- The structures of crystalline metals and simple ionic compounds can be described in terms of packing of spheres. Metal atoms can pack in primitive cubic, body-centered cubic, and face-centered cubic structures. Each packing has its own characteristics with respect to the volume occupied by the atoms and the closeness of the packing.
Glossary
- body-centered cubic (BCC) solid
1. crystalline structure that has a cubic unit cell with lattice points at the corners and in the center of the cell - body-centered cubic unit cell
1. simplest repeating unit of a body-centered cubic crystal; it is a cube containing lattice points at each corner and in the center of the cube