
What is the trend in ionic radius across a period?
4 rows · · Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its ...
What is the ionic radius?
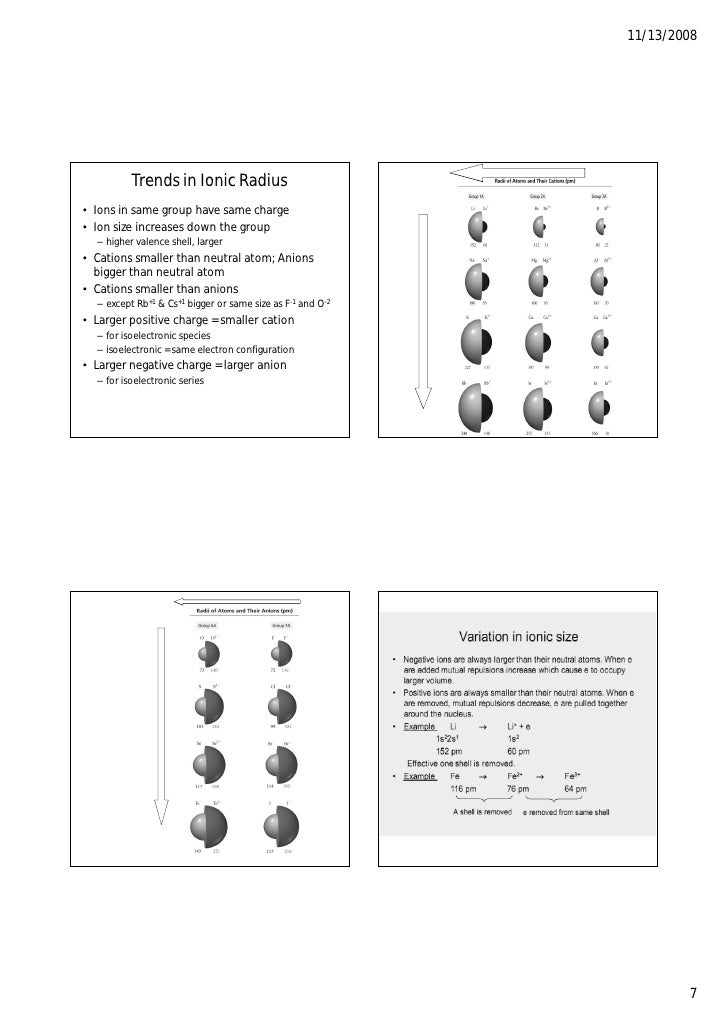
· Key Takeaways: Ionic Radius Trend on Periodic Table The ionic radius is half the distance between atomic ions in a crystal lattice. To find the value, ions are treated as if they were hard spheres. The size of an element's ionic radius follows a predictable trend on the periodic table. As you move down a column or group, the ionic radius increases.
Why does the ionic radius increase down a group?
· Ionic Radius Trend in the Periodic Table Ionic radius and atomic radius follow the same trends in the periodic table : As you move from top to bottom down an element group (column) ionic radius increases. This is because a new electron shell is added as you move down the periodic table. This increases the overall size of the atom.
What causes the trend in ionic radius?
The trend observed in size of ionic radii is due to shielding of the outermost electrons by the inner-shell electrons so that the outer shell electrons do not “feel” the entire positive charge of the nucleus.
How do you explain ionic radius?
Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Ions are formed when an atom loses or gains electrons. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion.
What is the trend for ionic radius for metals?
Ionic radii decreases across a period. This is due to the fact that metal cations lose electrons, causing the overall radius of an ion to decrease.
What are the trends for atomic and ionic radius?
Atomic radius increases going from top to bottom and decreases going across the periodic table. Ionic radius is the distance away from the central atom. Ionic radius increases going from top to bottom and decreases across the periodic table.
What is atomic radius trend?
Atomic Radius Trend on the Periodic Table. Atomic radii increase toward the bottom left corner of the periodic table, with Francium having the largest atomic radius. Atoms decrease in size across the period and increase in size down the group.
What ionic radius depends on?
Definition and Assumptions The ionic radius is similar to but different from the atomic radius for the ionic size is dependent on the distribution of its outermost electrons and is inversely proportional to the effective nuclear charge experienced by ions.
What is the trend across a period?
Moving from left to right across a period, atoms become smaller as the forces of attraction become stronger. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period.
What is the trend of ionization energy?
Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left to right across an element period. Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
What is the relationship of periodic trends and ionic compounds?
The elements on the left side of a period tend to form more ionic bonds, while those on the right side form more covalent bonds.
Why does atomic radius increase to the left?
On the periodic table, atomic radius generally decreases as you move from left to right across a period (due to increasing nuclear charge) and increases as you move down a group (due to the increasing number of electron shells).
What is atomic ionic radius?
Key Takeaways: Atomic vs Ionic Radius The atomic radius is half the diameter of a neutral atom. In other words, it is half the diameter of an atom, measuring across the outer stable electrons. The ionic radius is half the distance between two gas atoms that are just touching each other.
How do you compare ionic radius?
A comparison of ionic radii with atomic radii (Figure 8.2. 7) shows that a cation, having lost an electron, is always smaller than its parent neutral atom, and an anion, having gained an electron, is always larger than the parent neutral atom....Ionic Radii and Isoelectronic Series.IonRadius (pm)Atomic NumberAl3+57135 more rows•May 19, 2019
What is the ionic radius?
The ionic radius is half the distance between atomic ions in a crystal lattice. To find the value, ions are treated as if they were hard spheres.
What does the ionic radius of the elements exhibit?
She has taught science courses at the high school, college, and graduate levels. The ionic radius of the elements exhibits trends in the periodic table. In general: Ionic radius increases as you move from top to bottom on the periodic table.
What is the difference between ionic and atomic radius?
The ionic radius is different from the atomic radius of an element. Positive ions are smaller than their uncharged atoms. Negative ions are larger than their neutral atoms.
Why does the ionic radius decrease when you add more protons?
As you move across a row of the periodic table, the ionic radius decreases for metals forming cations, as the metals lose their outer electron orbitals. The ionic radius increases for nonmetals as the effective nuclear charge decreases due to the number of electrons exceeding the number of protons.
Why does radius increase with higher atomic numbers in a group?
Why does radius increase with higher atomic numbers in a group? As you move down a group in the periodic table, additional layers of electrons are being added, which naturally causes the ionic radius to increase as you move down the periodic table.
Why does the ionic radius increase as you move down a column?
As you move down a column or group, the ionic radius increases. This is because each row adds a new electron shell. Ionic radius decreases moving from left to right across a row or period. More protons are added, but the outer valence shell remains the same, so the positively charged nucleus draws in the electrons more tightly.
What increases as you move from top to bottom on the periodic table?
Ionic radius increases as you move from top to bottom on the periodic table.
What is the ionic radius?
The ionic radius (plural: ionic radii) is the measure of an atom's ion in a crystal lattice. It is half the distance between two ions that are barely touching each other. Since the boundary of the electron shell of an atom is somewhat fuzzy, the ions are often treated as though they were solid spheres fixed in a lattice.
How does ionic radius change?
Ionic radius and atomic radius follow the same trends in the periodic table : As you move from top to bottom down an element group (column) ionic radius increases. This is because a new electron shell is added as you move down the periodic table. This increases the overall size of the atom.
Why does the ionic radius decrease as you move down the periodic table?
This is because a new electron shell is added as you move down the periodic table. This increases the overall size of the atom. As you move from left to right across an element period (row) the ionic radius decreases. Even though the size of the atomic nucleus increases with larger atomic numbers moving across a period, ...
Why are ions smaller than atoms?
Cations are typically smaller than neutral atoms because an electron is removed and the remaining electrons are more tightly drawn in toward the nucleus. An anion has an additional electron, which increases the size of the electron cloud and may make the ionic radius larger than the atomic radius .
How to measure ionic radius?
Ionic radius can be measured with x-ray crystallography.
Do transition metals lose their outermost electrons?
The trend is particularly obvious with the metals, which form cations. These atoms lose their outermost electron, sometimes resulting in the loss of an entire electron shell. The ionic radius of transition metals in a period does not, however, change very much from one atom to the next near the beginning of a series.
Why does the atomic radius decrease with the size of the nucleus?
This is because the effective positive force of the nucleus also increases, drawing in the electrons more tightly. The trend is particularly obvious with the metals, which form cations.
What does the atomic radius trend mean?
The atomic radius trend reflects the change in the atomic radius that occurs as you follow the periodic table from top to bottom. The atomic radius of the different elements tends to increase as you move down the periodic table much as the ionic radius does.
What is the value of ionic radius?
The value of the ionic radius is half the distance between two ions which are just barely touching one another. An electron shell’s boundary is difficult to get an exact reading on, so the ions of an atom are typically treated as if they were solid spheres. The ionic radius can easily be a little smaller or larger than the atomic radius, ...
Why does the size of the atomic nucleus increase with the size of the atomic number?
Yet this occurs because as the size of the atomic nucleus increases so does the amount of positive force that has an effect on the atom’s electrons.
What is the radius of two atoms that are barely touching?
The atomic radius of two atoms that are barely touching is sometimes referred to as the van der Waals radius . This is because of the way that the week attraction of the van der Waals forces influences the distances of the two different atoms. The noble gas elements are frequently reported with the van der Waals radius .
Why do atomic radii change?
Take note that both the ionic radius and atomic radius aren’t fixed values. They can change because the various configurations of ions and atoms can influence the distance between the nuclei of the ions.
Is an ion larger than an atom?
Whether it is larger or smaller depends on the kind of electric charge the ion has. Cations, or positively charged ions, are usually smaller than a neutral atom of the element would be. This is because a cation has an electron removed from its shell, so the electrons which remain experience a greater attraction to the nucleus and are drawn towards it. In contrast, anions (negatively charged ions) have an additional electron. This electron increases the overall size of the electron cloud and can make the ionic radius bigger.
Is the ionic radius bigger than the atomic radius?
The ionic radius can easily be a little smaller or larger than the atomic radius, which is the radius a neutral atom of the element possesses. “A physicist is just an atom’s way of looking at itself.”. — Niels Bohr. Whether it is larger or smaller depends on the kind of electric charge the ion has.
What is the ionic radius trend?
The ionic radius trend refers to how the ionic radius of elements follows a predictable trend across the periodic table of the elements. Ionic radius tends to increase as you move from top to bottom down the periodic table, and it tends to decrease as you move left to right across the periodic table.
Why is ionic radius important?
Similarly, why is ionic radius important? What is the ionic radius of an ion and why is it important? For example, the ionic radius plays a large role in bonding; a larger ionic radius means more shielding (more inner shell electron repulsion) and thus a weaker bond, while a small ionic radius (something like Li+) would form stronger bonds.
Why do ionic radii decrease?
An ionic radius is defined as the radius of an atom's ion (ex. the radius of Na+). Ionic radii decrease across periods because effective nuclear charge increases. That is, the net positive charge experienced by an electron in the atom increases as a result of the number of protons in the nucleus increasing.
What is the ionic radius?
Hence, the ionic radius can be defined as the radial distance measured between the centre of the nucleus of an ion to the outermost electronic orbital where the electron cloud is still under the influence of the positive electric field of the nucleus.
What happens to the ionic radius when you move across a period?
So, the elements in the left typically form cations while those in the later periods towards the right form anions. Thus the ionic radius initially decreases and later increases, followed by another decrease.
What happens to the nature of an element when it moves across the periodic table?
While moving across any period in the periodic table, the nature of elements gradually changes from metallic to non-metallic. The number of electrons in the outermost shell keeps increasing until the last element (noble gas) is reached.
Why does the atomic radius decrease from left to right?
The atomic radius decreases from left to right across a period because the number of shells remains constant, but protonic charge keeps increasing , causing greater attractive pull on the outermost shell electrons. The ionic radius trend across a period varies accordingly, as mentioned above in the article.
What are periodic trends?
Ans: Periodic trends are patterns of change in properties (both chemical and physical) observed when we move across or down in the modern periodic table. The most common trends include atomic radius, ionic radius, electron affinity, electronegativity, ionization potential, etc.
Which is larger, nitride or oxide?
The ionic radius of Oxide (O2-) is larger than Nitride (N3-). The periodic table that we use in chemistry was first invented by Dmitri Mendeleev in the year 1869. It was in 1886 when Antoine Becquerel first discovered the concept of radioactivity.
Do cations have smaller radii than anions?
Following the periodic trend, cations have smaller radii than anions. Hence, we may conclude that ionic radius is an important periodic property whose trends can be monitored and usefully put to application in predicting properties of elements. Interestingly all periodic properties are interlinked and, to some extent, interdependent.
What are the trends in ionic radii?
Trends in Ionic Radii. Ions may be larger or smaller than the neutral atom, depending on the ion’s charge. When an atom loses an electron to form a cation, the lost electron no longer contributes to shielding the other electrons from the charge of the nucleus; consequently, the other electrons are more strongly attracted to the nucleus , ...
What is the ionic radius?
The ionic radius is the distance between the nucleus and the electron in the outermost shell of an ion. When an atom loses an electron to form a cation, the lost electron no longer contributes to shielding the other electrons from the charge of the nucleus; consequently, the other electrons are more strongly attracted to the nucleus, ...
Do ionic radii increase or decrease?
As with other types of atomic radii, ionic radii increase upon descending a group and decrease going across a period. Note that this only applies if the elements are the same type of ion, either cations or anions. For example, while neutral lithium is larger than neutral fluorine, the lithium cation is much smaller than the fluorine anion, due to the lithium cation having a different highest energy shell.
What are the colors of neutral atoms?
Sizes of atoms and their ions Relative sizes of atoms and ions. The neutral atoms are colored gray, cations red, and anions blue.
What is periodic trend?
In chemistry, periodic trends are the tendencies of certain elemental characteristics to increase or decrease along a period (row) or group (column) of the periodic table of elements. Ionic radius ( rion) is the radius of an ion, regardless of whether it is an anion or a cation. Although neither atoms nor ions have sharp boundaries, ...
Why do ionic radii increase?
The trend observed in size of ionic radii is due to shielding of the outermost electrons by the inner-shell electrons so that the outer shell electrons do not “feel” the entire positive charge ...
Is a cation positively charged?
cationA positively charged ion, as opposed to an anion. ionAn atom or group of atoms bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution. anionA negatively charged ion, as opposed to a cation.
What is ionic trend?
Ionic trend is in reference to the trend in ionic radii sizes throughout the periodic table of elements. While looking at a periodic table and moving down a group or column, the period number increases and therefore the atomic radius and ionic radius of those elements increases also.
How does ionic radius change?
Ionic radius describes the size of an ion depending on whether it gains or loses electrons. There is an atomic radius trend on the periodic table of elements where atoms with more orbitals have larger atomic radii, and atoms with more protons tend to have smaller atomic radii. When these atoms gain electrons, they become negatively charged ions called anions. Anions have larger ionic radii compared to the atomic radii of their parent atoms. When an atom loses an electron, it becomes a positively charged ion called a cation. Cations have smaller ionic radii compared to the atomic radii of their parent atoms. While looking at a periodic table of elements and moving from the top right toward the bottom left, the trend in atomic radius increases. This is because moving down a group increases the number of orbitals in the atom. Moving left across a period decreases the number of protons and reduces the positively charged influence on the orbitals of the electrons. Additionally, ionic radius trends loosely follow the same atomic radius trend on the periodic table, but ionic radius varies depending on how many electrons the atom gains or loses. Ionic radius is also dependent on the kinds of bonds surrounding the ion and the spin of the ion.
Why does the atomic radius increase when moving down a group?
Atomic radius increases moving down a group because the number of electron orbitals surrounding the nuclei of those atoms increases. Period numbers to the left of the periodic table indicate the number of electron orbitals neutral versions of those atoms in those elements possess. Moving down a group, period numbers increase. Therefore, the number of electron orbitals surrounding the nuclei increase, resulting in a larger atom; i.e., a larger atomic radius.
Why does the ionic radius increase as the group moves down?
Ionic radius increases moving down a group because the number of electron orbitals increases in number moving into higher and higher periods. Typically, an atom with a high number of electron orbitals will have a larger atomic radius, and therefore will likely have a large ionic radius compared to the elements above it in the same group.
How do atoms gain or lose electrons?
Whether an atom or ion gains or loses electrons depends on the elements it interacts with, the difference in electronegativity, and the ionization energy requirements between the different atoms or ions. Electronegativity increases in atoms moving from the bottom left toward the top right of the periodic table. Atoms at the top right of the periodic table have smaller amounts of orbitals surrounding their nuclei compared to their relatively more significant amounts of protons in their nuclei. These atoms have higher ionization energy requirements because of their smaller number of orbitals are greatly influenced by their relatively larger number of positively charged protons, pulling their electrons tighter around the nucleus. Therefore, they require greater energy to have their electrons stripped from them (i.e., ionization energy) because of their smaller atomic radii. These atoms tend to strip the electrons of atoms from the opposite side of the periodic table and increase their ionic radii. Overall, both atomic radius and ionic radius correlate with each other, and the number of protons in their nuclei and the number of electron orbitals surround their nuclei.
How do ionic and atomic radii differ?
Ionic radius is not a fixed property or stays the same as atomic radius does, but instead, it can vary due to the spin of the ion and its coordination number or the number of surrounding ions or atoms that are bonded to it. Ionic radii increase with the number of surrounding bonds or coordination because the charges of other ions or atomic nuclei influence the electron cloud and decrease its tightness around the central ion. Also, ionic radii increase with an increase in spin, i.e., the angular momentum and rotational flux of the ion forces the electron cloud to widen. If an ion is growing in positive charge, it loses electrons, and its ionic radius decreases. If an ion increases in negative charge, it is gaining electrons and increasing its atomic radius.
What is the ionic radius of an atom?
Ionic radius is the radius of an atom when it gains or loses an electron and becomes an ion. The periodic table also shows trends in the ionic radius of atoms as the ionic radius increases moving down a group. The chart under 'Ionic Radius vs. Atomic Radius' compares elements' atomic radii to their ionic radii.
What happens to the radius of a cation when we remove valence electrons?
But if we remove that valence electron, then inevitably the radius of the CATION should substantially decrease given that we have removed the shielding effect of an electron, and the nuclear charge contracts the radius of the remaining electrons...
Do metals tend to be oxidized?
As always, metals are electron-rich species, and TEND to be oxidized..whereas non-metals, high Z from the right of the Periodic Table as we face it..., TEND to be REDUCED to form anions...

Defining Ionic Radius
The Ionic Radius Trend
- The ionic radius trenddescribes the predictable pattern that the ionic radius follows as you move across the periodic table. As you follow the periodic table from top to bottom down a group of elements (a column) the ionic radius increases for the next element. This happens because elements further down the periodic table have more electron shells,...
Examples of The Ionic Radius Trend
- Let’s take a look at some examples of the ionic radius trend.Let’s examine the elements in Group 1 of the periodic table. 1. Lithium: Atomic Radius – 1.34 Sodium: Atomic Radius – 1.54 Potassium: Atomic Radius – 1.96 Rubidium: Atomic Radius – 2.11 Cesium: Atomic Radius – 2.25 Now let’s look at some of the elements within Group 17 of the periodic table: 1. Fluorine: Atomic …
Other Trends in The Periodic Table
- The atomic radius trend reflects the change in the atomic radius that occurs as you follow the periodic table from top to bottom. The atomic radius of the different elements tends to increase as you move down the periodic table much as the ionic radius does. Theelectronegativity trenddescribes how as one follows the periodic table left to right electronegativity tends to incre…