
As well as being important in analytical chemistry, the precipitation of silver phosphate is also used in silver staining of biological materials (after reduction to silver metal) - as a magnifying agent for phosphate. Silver phosphate also found use in early photography as a light sensitive agent.
What is silver phosphate used for in photography?
Silver phosphate also found use in early photography as a light sensitive agent. In 2010, silver phosphate was reported as having a high (90%) quantum yield as a photocatalyst for the visible light photochemical splitting of water, and for production of activated oxygen by the same method.
What is the use of silver phosphate precipitate?
The precipiation of silver phosphate is useful in traditional analytical chemistry. Precipitation of silver phosphate is also used in silver staining of biological materials (after reduction to silver metal) - as a magnifying agent for phosphate. [9] Silver phosphate also found use in early photography as a light sensitive agent. [10]
Is silver phosphate water soluble?
Ask an American Elements Materials Science Engineer Silver Phosphate is a moderately water and acid soluble Silver source for uses compatible with Phosphates. Silver Phosphate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.
What is the formula for silver phosphate?
Silver Phosphate Formula: Structure, Properties ... Silver Phosphate formula is an inorganic salt created by one phosphate anion and three silver cations. It is generally available in most volumes. The chemical or molecular formula of silver phosphate is Ag 3 PO 4.

Silver Phosphate
Silver phosphate is a yellow, water-insoluble compound. It is also a light sensitive chemical compound composed of silver and phosphate ions. The compound is formed as a yellow precipitate due to the reaction between a soluble silver compound with a soluble orthophosphate.
Silver Phosphate Structure
The chemical formula of silver phosphate is Ag?PO?. It is a yellow solid in its pure form and becomes darker in its impure form. The formula structure of silver phosphate is given below.
Properties of Silver Phosphate
The molar mass of silver phosphate is 418.574 g mol?¹ and the density is 6.37 g mL?¹.
Uses of Silver Phosphate
Silver phosphate is used as catalysts such as bactericide, astringent, etc in some medical purposes and used in the staining of biological materials.
Things to Remember
Silver phosphate is a light sensitive chemical compound made of silver and phosphate ions. The chemical formula of silver phosphate is Ag?PO?.
Sample Questions
Ag?PO?. Silver phosphate or silver orthophosphate is a yellow compound composed of silver and phosphate ions.
What is silver phosphate used for?
As well as being important in analytical chemistry, the precipitation of silver phosphate is also used in silver staining of biological materials (after reduction to silver metal) - as a magnifying agent for phosphate.
What is the melting point of silver pyrophosphate?
Like silver orthophosphate it is light sensitive. Silver orthophosphate turns red on exposure to light. It has a density of 5.306 g/cm 3 and a melting point of 585 °C. A hydrate also exists which decomposes at 110 °C.
Is silver phosphate a light sensitive agent?
Silver phosphate also found use in early photography as a light sensitive agent. In 2010, silver phosphate was reported as having a high (90%) quantum yield as a photocatalyst for the visible light photochemical splitting of water, and for production of activated oxygen by the same method.
About Silver Phosphate
Silver Phosphate is a moderately water and acid soluble Silver source for uses compatible with Phosphates. Silver Phosphate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.
Packaging Specifications
Typical bulk packaging includes palletized plastic 5 gallon/25 kg. pails, fiber and steel drums to 1 ton super sacks in full container (FCL) or truck load (T/L) quantities. Research and sample quantities and hygroscopic, oxidizing or other air sensitive materials may be packaged under argon or vacuum.
Recent Research
Nitrogen doped carbon quantum dots mediated silver phosphate/bismuth vanadate Z-scheme photocatalyst for enhanced antibiotic degradation.
What is silver phosphate?
Silver phosphate, also known argentous phosphate or silver orthophosphate, is an inorganic salt used as stained or light sensitive substance for revealing in photography.
What is the melting point of silver phosphate?
Silver phosphate melting point is 849 °C. It is light sensitive. Chemical properties: The ion phosphate at high temperature can suffer a reaction of condensation to form pyrophosphate, in these conditions it is also possible to form silver pyrophosphate when silver nitrate is added.
Is silver phosphate found in nature?
Occurrence: Silver phosphate cannot be found in nature. It is prepared as described below.
Is silver phosphate a catalyst?
Moreover, it can be used as a staining of biological materials. Due to silver phosphate is a light sensitive agent; it is used in photography as emulsions. It is also used as catalysts on some reactions and for medical purposes as astringent, bactericide, etc.
What is colloidal silver?
Colloidal silver is the term used to describe tiny particles of silver suspended in a liquid. Given their small size, a normal filtering process would not remove them. The size of the silver particles in colloidal silver can vary, but some are so tiny that they are referred to as “nanoparticles.”. This means that they are less than 100 nm in size ...
What happens when silver ions pass through a cell?
This allows silver ions to pass into the cells, where they can interfere with the bacteria’s metabolic processes and damage its DNA, leading to the cell’s death.
What is the silver ion?
Silver ions are released from the silver particles when they come into contact with moisture, such as body fluids. They are considered to be the “biologically active” part of colloidal silver that gives it its medicinal properties ( 4. Trusted Source. , 7, 8. Trusted Source.
What is the biggest risk associated with colloidal silver exposure?
The biggest risk associated with chronic exposure to colloidal silver is argyria.
Does colloidal silver damage DNA?
The exact way colloidal silver works is not fully understood. However, it’s thought that silver can bind to bacterial cells and damage their cell walls and DNA, resulting in cell death.
Can colloidal silver be used for lyme disease?
A smaller number of people also claim it can help treat illnesses such as Lyme disease, tuberculosis and even HIV/AIDs. Those who use colloidal silver take it as a dietary supplement or apply it directly to their skin. The solution can be found in various strengths, depending on how much silver it contains. Summary.
Is colloidal silver a good antifungal?
At the moment, there is little evidence to support the claim that colloidal silver is a reliable topical antifungal agent. Additionally, no studies have investigated the effects of ingesting colloidal silver on fungal infections in humans. Summary.
What is silver used for?
Silver is generally known for its use in coins, silverware and of course jewellery. However, today these account for less than 50% of all silver consumption. In fact, silver has a whole host of unique properties that has rendered it the ideal material for a number of industrial uses. Of all the metals, silver is the best thermal ...
Is silver a good anti-microbial?
For these reasons it is extremely valuable in industrial and electrical applications. On top of this, silver is anti-microbial as well as non-toxic which makes it useful in medicine and a number of consumer products. Also, with the recent discovery of nano-silver, the white metal has expanded into home appliances.
Is silver cheap?
In comparison to most other precious metals, silver is more widely available and relatively cheap. In many instances this has led to silver being seen as disposable with nano-silver being used in a plethora of everyday items – who would have thought we’d consider washing our clothes with nano-particles of the metal we once wore as prized jewellery?
Is silver a good conductor?
Of all the metals, silver is the best thermal and electrical conductor, is both malleable and ductile, able to be flattened into fine sheets and drawn out into thin, flexible wires, and it is also resistant to corrosion and oxidation. For these reasons it is extremely valuable in industrial and electrical applications.
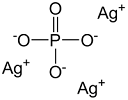
Overview
Silver phosphate or silver orthophosphate is a light sensitive, yellow, water-insoluble chemical compound composed of silver and phosphate ions of formula Ag3PO4.
Synthesis, reactions and properties
Silver phosphate is formed as a yellow solid precipitate by the reaction between a soluble silver salt, such as silver nitrate, with a soluble orthophosphate. Its solubility product is 8.89×10 mol ·dm . The precipitation reaction is analytically significant and can be used in qualitative or quantitative and quantitative analysis.
This compound dissolves in aqueous ammonia. Large crystals of silver phosphate form upon gr…
Uses
The precipitation of silver phosphate is useful in traditional analytical chemistry. Precipitation of silver phosphate is also used in silver staining of biological materials (after reduction to silver metal) - as a magnifying agent for phosphate.
Silver phosphate also found use in early photography as a light sensitive agent.
Silver phosphate exhibits antibacterial properties.
Research
Silver phosphate is a high (90%) quantum yield photocatalyst for the visible light photochemical splitting of water and for production of activated oxygen by the same method.
Other silver phosphates
Silver pyrophosphate Ag4P2O7 (CAS No. 13465-97-9) can be prepared as a white precipitate from reaction of silver(I) and pyrophosphate ions. Like silver orthophosphate it is light sensitive. Silver orthophosphate turns red on exposure to light. It has a density of 5.306 g/cm and a melting point of 585 °C. A hydrate also exists which decomposes at 110 °C.
Silver metaphosphate (AgPO3) (CAS No. 13465-96-8) is a white solid with a density of 6.370 g/c…