Why does H2S have a lower boiling point than H2Se?
This essentially means H2Se is a bigger molecule than H2S meaning the Van Der Waal forces (London forces) on H2Se is stronger than on H2S which means it takes more energy to break the intermolecular forces for H2Se than H2S- this leads to H2S having the lower boiling point. However a conflicting idea is that sulfur is more electronegative than selenium so you may think the permanent dipole dipole forces will be stronger for H2S and you are right.
Why boiling point of water is higher than H2S?
Why is boiling point of h20 higher than H2S? Water has a higher boiling point than hydrogen sulphide. The intermolecular attractions between water molecules are stronger than H2S molecules due to hydrogen bonding in H2O due to high electronegativity and small size of oxygen atom.
Which has the highest boiling point H2S or H2Se?
H2Te has the highest molecular weight and thus it has the highest boiling point from H2S and H2Se. Which is most acidic among H2O H2S H2Se H2Te? In binary acids such as H2S and H2Se, the H–Se bonds is longer than the H–S bonds as Se is larger than S.
What are the hazards of H2S?
Hydrogen sulfide is a chemical compound with the formula H. 2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp, which is ...

What is the chemical name for hydrogen sulfide?
Hydrogen sulfide is a chemical compound with the formula H#N#2S. It is a colorless chalcogen hydride gas with the characteristic foul odor of rotten eggs. It is poisonous, corrosive, and flammable.
What is the reaction of sulfur dioxide and hydrogen sulfide?
At high temperatures or in the presence of catalysts, sulfur dioxide reacts with hydrogen sulfide to form elemental sulfur and water. This reaction is exploited in the Claus process, an important industrial method to dispose of hydrogen sulfide. ).
How is 2S converted to sulfur?
2S is converted to elemental sulfur by partial combustion via the Claus process, which is a major source of elemental sulfur. Other anthropogenic sources of hydrogen sulfide include coke ovens, paper mills (using the Kraft process ), tanneries and sewerage. H.
How do metals react with hydrogen sulfide?
As indicated above, many metal ions react with hydrogen sulfide to give the corresponding metal sulfides. This conversion is widely exploited. For example, gases or waters contaminated by hydrogen sulfide can be cleaned with metals, by forming metal sulfides. In the purification of metal ores by flotation, mineral powders are often treated with hydrogen sulfide to enhance the separation. Metal parts are sometimes passivated with hydrogen sulfide. Catalysts used in hydrodesulfurization are routinely activated with hydrogen sulfide, and the behavior of metallic catalysts used in other parts of a refinery is also modified using hydrogen sulfide.
How is hydrogen sulfide obtained?
Hydrogen sulfide is most commonly obtained by its separation from sour gas, which is natural gas with a high content of H#N#2S. It can also be produced by treating hydrogen with molten elemental sulfur at about 450 °C. Hydrocarbons can serve as a source of hydrogen in this process.
Which bacteria use hydrogen sulfide as an electron donor in photosynthesis?
The purple sulfur bacteria and the green sulfur bacteria use hydrogen sulfide as an electron donor in photosynthesis, thereby producing elemental sulfur. This mode of photosynthesis is older than the mode of cyanobacteria, algae, and plants, which uses water as electron donor and liberates oxygen.
Where does hydrogen sulfide come from?
MS + H#N#2O → MO + H#N#2S. Hydrogen sulfide can be present naturally in well water, often as a result of the action of sulfate-reducing bacteria. Hydrogen sulfide is created by the human body in small doses through bacterial breakdown of proteins containing sulfur in the intestinal tract, therefore it contributes to the characteristic odor of flatulence. It is also produced in the mouth ( halitosis ).
What is the phase diagram of hydrogen sulfide?
The hydrogen sulfide phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the hydrogen sulfide boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
What is hydrogen sulfide used for?
Hydrogen sulfide is used in the manufacture of chemicals, in metallurgy, and as an analytical reagent.
What is the triple point of a substance?
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. Back to top.
Is a sulfate reagent heavier than air?
It is used in the manufacture of chemicals, in metallurgy, and as an analytical reagent. It is heavier than air and tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first, it quickly deadens the sense of smell.
Is H2S a toxic gas?
Phase diagram included. Hydrogen sulfide, H2S, is a highly toxic and flam mable, color less gas with a characteristic odor of rotten eggs.
Is hydrogen sulfide a liquid or solid?
However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The hydrogen sulfide phase diagram shows the phase behavior with changes in temperature and pressure.
Why is the boiling point of H2O higher than that of H2S?
It's due to the higher electronegativity of Oxygen than Sulphur that it makes Intermolecular Hydrogen bonds with the Hydrogen Atoms of Other water molecules thus the Boiling Point of H2O increases and is much higher than that of H2S.
Why is H20 a high boiling point?
The reason that h20 has a high boiling point is because of an intermolecular force called hydrogen bounding ( which is separate from the OH bonds) When water is boiled the OH bonds are not broken, the water molecules just move away from one another enough that they become a gas.
How is hydrogen bond formed?
Hydrogen bond is formed between two molecules if they have hydrogen and any of the three electronegative atoms (N,O,F) covalently bonded to each other . As there is no (NOF) in H2S , there is no hydrogen bond there although it has dipole dipole forces.
What is the difference between a water molecule and an oxigen molecule?
The two molecules has the same geometrical form, the only difference is the smaller and higher electronegativity O atom. Due to its higher electronegativity, The electrons of the H atoms spend more time around the oxigen, making slightly dipole the whole molecule. The "positive" side, with the hidrogens attracted to the other water-molecules oxigen atoms, creating a weak H- or Van der Waals bond. These connected water molcules creating tangled water chains and required more energy to break apart.
Why does the angle of the bond increase in H2O?
Consequently more bond pair- bond pair repulsion take place,so bond angle get increase. On the other hand in H2S, because of less electronegative character of S,less repulsion is present so angle get decrease.
How many electrons are in the outer shell of sulfur?
The atomic structure of the central atoms means that the oxygen and sulfur both have 6 electrons in their outermost shell. Two of those electrons are donated to the bonds to the two hydrogen atoms in the molecule, one donated to each bond. The remaining four electrons are therefore considered “spare” for interacting with other molecules.
Which molecule has the lowest boiling point?
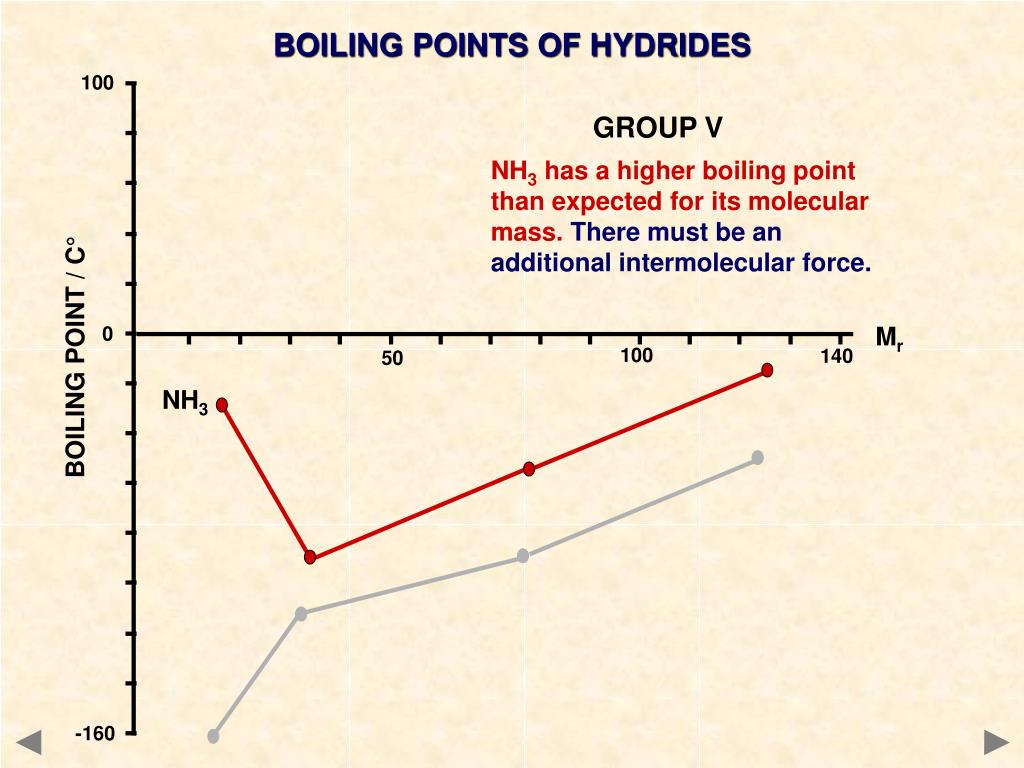
H2S has the lowest boiling point. As the molar mass increases boiling point of the hydrides increases except H2O. The exception of water is due to intermolecular hydrogen bonding among the water molecules. Water molecules are in associated state. The strong hydrogen bonding is formed due to high electronegativity and extremely smaller size of oxygen. The high ‘O—H’ bond polarity is effective for strong H-bonding. Decreasing order of boiling point: H2O > H2Te > H2Se > H2S.
H2S and H2Se Relative Boiling Points
Hello! This may be a dumb question, but I was wondering about this question from Matthew Nguyen's UA Worksheets?
Re: H2S and H2Se Relative Boiling Points
When looking at what has a higher boiling point, we look at what has the strongest intermolecular forces. They both have dipole-dipole which I think are the same strength. I'm not sure if the higher electronegativity of S would make the dipole-dipole interaction stronger.

Overview
Safety
Hydrogen sulfide is a highly toxic and flammable gas (flammable range: 4.3–46%). Being heavier than air, it tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first (it smells like rotten eggs ), it quickly deadens the sense of smell, creating temporary anosmia, so victims may be unaware of its presence until it is too late. Safe handling procedures are provided by its safety data sheet (SDS).
Properties
Hydrogen sulfide is slightly denser than air. A mixture of H 2S and air can be explosive. Hydrogen sulfide burns in oxygen with a blue flame to form sulfur dioxide (SO 2) and water. In general, hydrogen sulfide acts as a reducing agent, although in the presence of a base, it can act as an acid by donating a proton and forming SH .
At high temperatures or in the presence of catalysts, sulfur dioxide reacts with hydrogen sulfide t…
Production
Hydrogen sulfide is most commonly obtained by its separation from sour gas, which is natural gas with a high content of H 2S. It can also be produced by treating hydrogen with molten elemental sulfur at about 450 °C. Hydrocarbons can serve as a source of hydrogen in this process.
Sulfate-reducing (resp. sulfur-reducing) bacteria generate usable energy under low-oxygen conditions by using sulfates (resp. elemental sulfur) to oxidize organic compounds or hydrogen; t…
Uses
The main use of hydrogen sulfide is as a precursor to elemental sulfur. Several organosulfur compounds are produced using hydrogen sulfide. These include methanethiol, ethanethiol, and thioglycolic acid.
Upon combining with alkali metal bases, hydrogen sulfide converts to alkali hydrosulfides such as sodium hydrosulfide and sodium sulfide:
Hydrogen sulfide in the natural environment
Hydrogen sulfide is a central participant in the sulfur cycle, the biogeochemical cycle of sulfur on Earth.
In the absence of oxygen, sulfur-reducing and sulfate-reducing bacteria derive energy from oxidizing hydrogen or organic molecules by reducing elemental sulfur or sulfate to hydrogen sulfide. Other bacteria liberate hydrogen sulfide fr…
See also
• Hydrogen chalcogenide
• Hydrogen sulfide chemosynthesis
• Jenkem – Type of street drug
• Sewer gas
Additional resources
• Committee on Medical and Biological Effects of Environmental Pollutants (1979). Hydrogen Sulfide. Baltimore: University Park Press. ISBN 978-0-8391-0127-7.
• Siefers, Andrea (2010). A novel and cost-effective hydrogen sulfide removal technology using tire derived rubber particles (MS thesis). Iowa State University. Retrieved 8 February 2013.