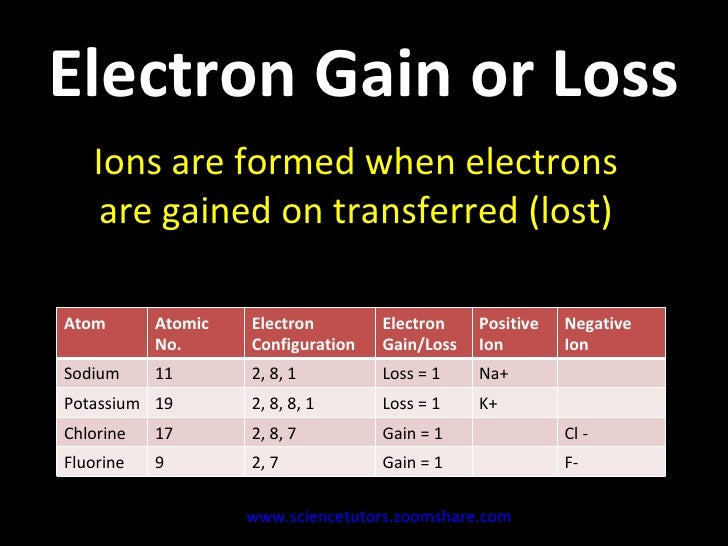
What is an atom that loses an electron called?
An atom that loses an electron is called a positive ion. An electron is a negatively charged particle, and the atom becomes positively charged upon its removal. An atom that loses an electron has more protons than electrons. An atom that gains an electron adds a negative charge...
What happens when an electron is removed from an atom?
An electron is a negatively charged particle, and the atom becomes positively charged upon its removal. An atom that loses an electron has more protons than electrons. An atom that gains an electron adds a negative charge and becomes a negative ion.
What happens when an atom gains an electron?
It remains neutral because the proton also leaves. It stays the same. Q. What happens when an atom gains an electron? It remains neutral because the proton also leaves. It stays the same. Quiz not found!

What is the charge of an atom that has gained 1 electron?
Atoms that gain extra electrons become negatively charged. A neutral chlorine atom, for example, contains 17 protons and 17 electrons. By adding one more electron we get a negatively charged Cl- ion with a net charge of -1.
What is the charge of an atom that has lost one electron quizlet?
When an atom loses one of its electrons, it becomes a positively charged ion. The atom that gains the electron becomes a negatively charged ion.
When an atom loses an electron what happens?
Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations.
When an atom loses one or more electrons it is said to be quizlet?
an atom that loses one or more electrons from its outermost energy level becomes a positively charged ion.
What happens when an atom loses an electron Quizizz?
What happens when an atom loses an electron? It remains neutral because the proton also leaves.
Are charged atoms that have gained or lost one or more electrons?
Definition: An ion is an atom or molecule which has lost or gained one or more electrons, and carries a net electric charge. If the result is an atom with more electrons than protons, the ion is negatively charged.
What atom has gained or lost an electron?
An Ion is defined as an atom which has gained or lost electrons, thus it possesses an overall charge.
What are negative ions called?
ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
What is it called when an atom loses an electron?
An atom that loses an electron is called a positive ion. An electron is a negatively charged particle, and the atom becomes positively charged upon its removal. An atom that loses an electron has more protons than electrons. An atom that gains an electron adds a negative charge and becomes a negative ion. A negative ion contains more electrons ...
Which atom has a negative charge?
An atom that gains an electron adds a negative charge and becomes a negative ion. A negative ion contains more electrons than protons, resulting in the negative charge. Sodium, potassium, calcium and iron are common examples of positive ions. Chloride and phosphorous are two types of negative ions. A molecule such as sodium chloride is ...