
What is the potential energy of a reaction measured in?
The potential energy of a reaction measures the energy stored within the bonds and phases of the reactants and products. When a reaction occurs, there is a change in the amount of potential energy stored in the bonds between the reactants and the products. The difference in this potential energy is referred to as enthalpy.
What is difference between potential energy and enthalpy change?
Still stuck? Get 1-on-1 help from an expert tutor now. In a chemical reaction, the difference between the potential energy of the products of the potential energy of the reactants is equal to the enthalpy change. What is potential energy of a reaction?
What is meant by potential energy?
Potential Energy is the energy stored in a system when work is done on it by some external agency. Potential is the work done per unit something of that system e.g. electric potential is just work done per unit charge. Similarly, gravitational potential is work done per unit mass.
What is the potential energy of an object at infinity?
At infinity, the potential energy of something is zero. But when we think about it, as you raise an object higher, further from the surface of the Earth, it is said that the potential energy increases, and at the surface, you don't have any potential energy, as when it falls, it's converted into kinetic energy.
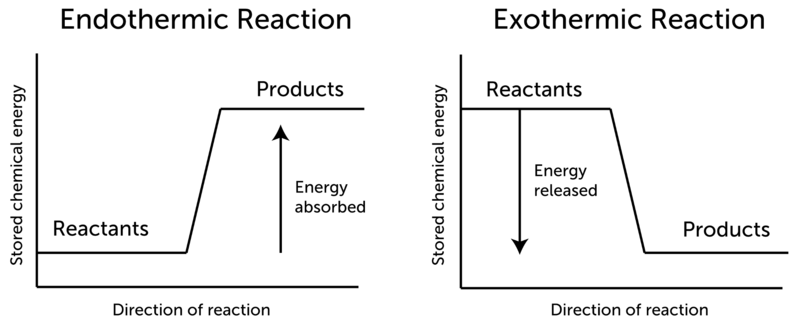
What does this mean for the potential energy of the reactants and products?
1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. ( A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (
How do you know if reactants or products have more potential energy?
3:0911:31Potential Energy Diagrams - Catalyst, Endothermic & Exothermic ReactionsYouTubeStart of suggested clipEnd of suggested clipYou need to draw the products with a higher energy than reactants. So the products have to be aboveMoreYou need to draw the products with a higher energy than reactants. So the products have to be above the reactants.
In which reaction do the reactants have a lower potential energy than the products?
An exothermic reaction is one in which heat energy is given out. The products must have less energy than the reactants because energy has been released. This can be shown by a potential energy diagram: For exothermic reactions, will always be negative.
Is the potential energy of the reactants higher or lower than the potential energy of the products in an exothermic reaction?
(B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is negative. The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products.
How does the energy of the reactants compared with the energy of the products?
The difference between the energy of the reactants and the energy of the products is called the enthalpy change (∆H) of the reaction. For an exothermic reaction, the enthalpy change is always negative. In an endothermic reaction, the products are at a higher energy than the reactants.
Why do reactants have more energy than products?
Energy Diagrams In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products.
How does the potential energy of reactants compare to the energy of products in an endothermic reaction an exothermic reaction?
Exothermic reactions have a potential energy difference between the products and reactants that is negative. Endothermic reactions have a potential energy difference between the products and reactants that is positive.
Which is true about the potential energy of the reactants in this reaction pathway?
Which is true about the potential energy of the reactants in this reaction pathway? It is equal to the potential energy of the products.
In which type of reaction is there more energy in the products than in the reactants?
In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants.
Which is higher in an endothermic reaction the potential energy of the reactants or the potential energy of the products?
In an endothermic reaction the potential energies of the reactants are lower than the products.
Do reactants or products have more energy in an endothermic reaction?
In an endothermic reaction, the products are at a higher energy than the reactants. This means that the enthalpy change of the reaction (∆H) is positive.
How does having a higher potential energy for the reactant affect the activation energy?
This is where an increase in potential energy begins in reactant molecules. The faster the motion of the molecules originally was, the greater the increase in potential energy this will cause. If this rise in potential energy does not match the activation energy, nothing will happen.
Which molecule has a higher potential energy?
Which molecule, ATP or ADP, have a higher potential energy? ATP because it has three phosphate groups rather than ADP which has two.
Which molecule has more potential energy?
a) Glucose contains the most potential energy.
What does it mean for a chemical to have a low potential energy?
In chemicals, potential energy is stored in form of chemical bonds. So, if a chemical have lesser potential energy, means its bonds have lesser energy and to break these bond higher amount of energy is required.
What is potential energy in a reaction?
Potential Energy is the energy due to position, composition, or arrangement. Also, it is the energy associated with forces of attraction and repulsion between objects.