What is the Lewis electron dot structure?
The representation of molecules in Lewis electron dot structure is in the honor of the American chemist G N Lewis. Electron dot structures are the diagrams to describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs available in each of the atoms of the molecule. How to Draw Electron Dot Formula?
How do you draw an electron dot structure?
We can draw it if we know the molecular formula of the compound. It is also useful to represent the atomic state of elements. We represent an electron dot structure by using the element symbol. Start from the top of the element symbol, then add dots in a clockwise manner to complete the number of valence electrons.
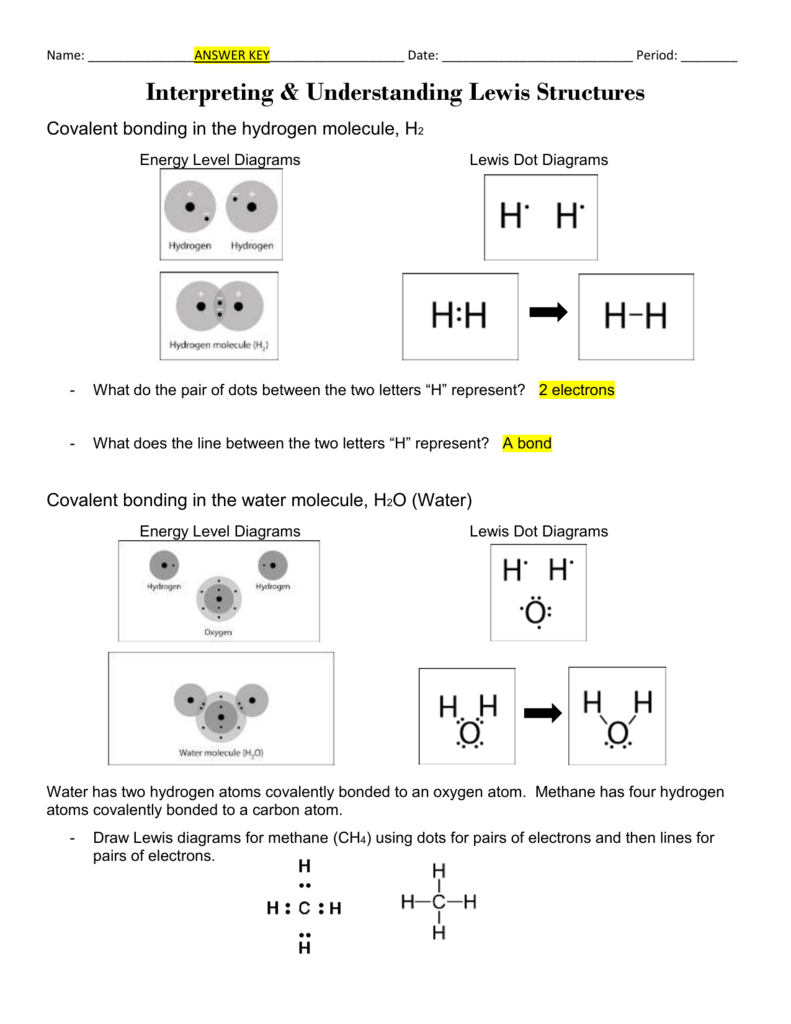
What is Lewis structure of H2O?
Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial. H 2 O lewis structure
How many valence electrons are there in 1 electron dot?
For example, the elements in group IA of the chemical periodic table have 1 valence electron. In chemistry electron dot formula has its own importance. This article will explain the electron dot formula with examples.

What is the correct electron dot formula for water?
Therefore, a water molecule has 2 bond pairs of electrons and 2 lone pair (non-bonded) pairs of electrons. Therefore, corresponding to the question, we can that the correct Lewis dot structure of water molecules is number 3. So, Option C is the correct answer.
What is an electron dot formula?
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
How do you write a dot structure?
0:266:48Trick To Draw Lewis Dot Structures - YouTubeYouTubeStart of suggested clipEnd of suggested clipStep number four complete octet with remaining electrons for the outer atoms first and add remainingMoreStep number four complete octet with remaining electrons for the outer atoms first and add remaining electrons to the central atom. Step number five complete the central atom update.
How do you draw a dot structure?
1:237:26Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou put the dots it doesn't really matter as long as you neatly draw them along the sides of anMoreYou put the dots it doesn't really matter as long as you neatly draw them along the sides of an imaginary Square.
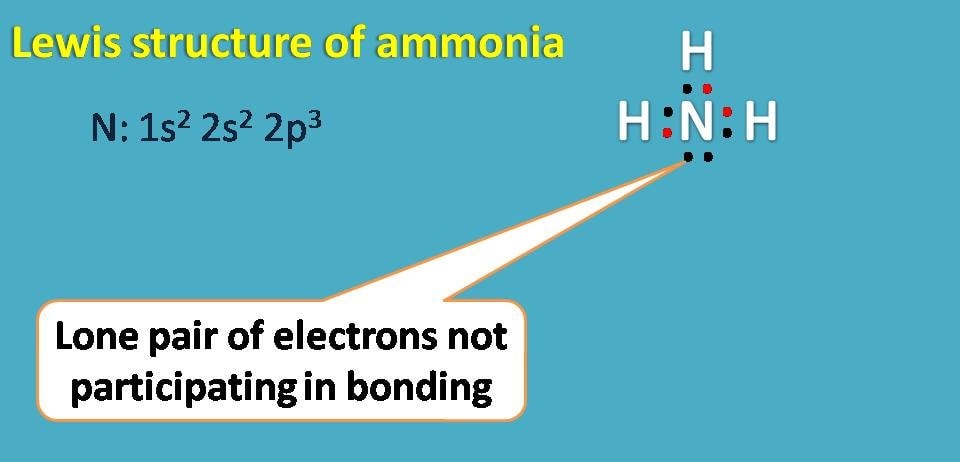
What is the electron dot structure for CH4?
The electron dot structures are used to show the bond formation among the elements of a covalent chemical compound. Similarly, the CH4 is also a covalent compound. There are 4 valence electrons of the central carbon atom and each of the four hydrogen atoms have 1 valence electrons.draw the electron dot structure of CH4 - Brainly.inhttps://brainly.in › questionhttps://brainly.in › questionSearch for: What is the electron dot structure for CH4?
How do you write an electron dot structure for Class 10?
To write the electron dot structure, put two hydrogen atoms at terminal positions and two carbon atoms in the internal positions. All the four atoms are placed in one line. Now place one covalent bond between two carbon atoms and place two covalent bonds between carbon and hydrogen atoms.Draw electron dot structure of ethyne class 10 chemistry CBSE - Vedantuhttps://www.vedantu.com › question-answerhttps://www.vedantu.com › question-answerSearch for: How do you write an electron dot structure for Class 10?
How to Draw Electron Dot Formula?
Electron Dot Formula comprises of one dot for every valence electron with the element’s symbol. Note down that a skeletal structure displaying a realistic bonding pattern by means of only the element symbols is very important.
What is the Lewis electron dot structure?
Electron dot structures are the diagrams to describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs available in each of the atoms of the molecule.
How to find the total number of valence electrons in a molecule?
First, calculate the total number of valence electrons present in the molecule by adding the individual valences of each atom.
What bond should be used to satisfy the octet valency of each atom?
After this, if every atom does not have an octet configuration, then a double or triple bond should be used to satisfy the octet valency of each atom. At last, if required, we have to convert a lone pair into a bond pair in order to satisfy the octet rule for two atoms.
Which atom has 6 valence electrons?
Q.1: Explain the Electron Dot Formula of. Solution: The central atom of this molecule is the carbon atom. Oxygen contains 6 valence electrons with 2 lone pairs. Then, it is bonded to only one carbon atom with a double bond. Carbon contains four valence electrons, therefore giving zero lone pairs. So, it is doubly bonded to each oxygen atom.
Where is the least electronegative atom?
The least electronegative atom is at the central atom of the molecule or ion.
How to find total electron pairs?
Total electron pairs are determined by dividing the number total valence electrons by two. For, H 2 O, Total pairs of electrons are 4 in their valence shells.
How many electron pairs are there in a H-O bond?
Remember that, there are total of four electron pairs. There are already two H-O bonds in the drawn sketch structure. Now <>only two (4-2) electron pairs are remaining to mark on atoms. Usually, those remaining electron pairs should be started to mark on outside atoms.
What are the other ways drawing lewis structure for water?
In this tutorial, we took total electrons in last shells of elements ( oxygen and hydrogen atoms). Instead of that, we can valence of oxygen is two and those two electrons should be used to make bonds with two hydrogen atoms.
What are the similar lewis structures to water can be drawn for?
In lewis structure of water molecule, there are two sigma bonds and two lone pairs around sulfuratom. Hydrogen sulfide, oxygen difluoride (F 2 O) have similar lewis structures to water.
How many electrons are in the last shell of hydrogen?
Hydrogen is a group IA element and has only one electron in its last shell (valence shell). Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. Now we know how many electrons are includes in valence shells of each atom. valence electrons given by hydrogen atoms = 1 * 2 = 2.
How to check the stability and minimize charges on atoms?
Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
How many bonds does Lewis structure have?
Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
What is the chemical formula for water?
Water (chemical formula: H2O) is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds.
What are the reactions of water?
Water reacts with many substances, including but not limited to alkali metals, hydrides, strong halogenating agents, and chlorosilanes. These reactions can be hazardous and may result in flammable or toxic gas production, or generation of excessive heat that may cause pressurization to occur. Another reactive hazard is heat of mixing. Mixing substances such as sulfuric acid or sodium hydroxide with water may generate significant heat. Additionally, water is a good solvent for polar molecules, so it can form aqueous solutions if it comes into contact with such molecules.
Do bottled water have fluoride?
Some bottled waters contain fluoride, and some do not. Fluoride can occur naturally in source waters used for bottling or be added. Most bottled waters contain fluoride at levels that are less than optimal for good oral health.
Is water a non-toxic liquid?
Water appears as a clear, nontoxic liquid composed of hydrogen and oxygen, essential for life and the most widely used solvent. Include water in a mixture to learn how it could react with other chemicals in the mixture.
Is water a toxic substance?
Water itself is nontoxic and is in fact essential for life. Solutes dissolved in water may be toxic, but those interactions are covered by the reactive groups that the solute belongs to.