Which is described as the force holding two atoms together?
The forces that hold atoms together are the electrical force and the strong force, which is stronger than the electrical force. The electrical force does the majority of the work of holding atoms together, but the strong force helps hold in the electrical force and can somewhat override it.
Which two forces are responsible for holding the atom together?
- The strong forces oppose the electromagnetic force of repulsion between protons. ...
- The strong forces and electromagnetic forces both hold the atom together. ...
- Weak forces are important because they are responsible for stabilizing particles through the process of radioactive decay, in which a neutron in the nucleus changes into a proton and an ...
What force binds the atoms together?
Atoms have different features that single out one atom from another, and show how each atom can change in different conditions. These properties include atomic number, mass number, atomic mass and weight, and isotopes.. Forces acting. In an atom there are three fundamental forces that keep atoms together. electromagnetic force, strong nuclear force, and weak nuclear force.
What forces hold the various parts of an atom together?
· The strong forces and electromagnetic forces both hold the atom together. · Weak forces are important because they are responsible for stabilizing particles through the process of radioactive decay, in which a neutron in the nucleus changes into a proton and an electron.
What forces hold atoms together?
The forces that hold atoms together are the electrical force and the strong force, which is stronger than the electrical force. The electrical force does the majority of the work of holding atoms together, but the strong force helps hold in the electrical force and can somewhat override it. The electrical charge that is exhibited from protons ...
Why does the strong force work against itself?
It also works against itself because two protons and two electrons repel each other, even though they are pulling closer together through the electrical charge. The strong force is able to remedy the problem of the electrical charge not being strong enough to hold everything together. It is stronger than the electrical force ...
Why does the charge of an atom make it stronger?
The electrical charge that is exhibited from protons and electrons attracting to each other causes the force of the atoms to become stronger. The charge sets up the framework for the electrical force that is used to hold everything together, but it is not strong enough to keep the atoms close enough together. While the force helps electrons and protons attract to each other, it does nothing for the neutrons, which are neutral. It also works against itself because two protons and two electrons repel each other, even though they are pulling closer together through the electrical charge.
Is the force of a neutron stronger than the force of an atom?
It is stronger than the electrical force and helps to hold all of the protons and neutrons in, which are immune to the electrical force. It is a very strong force, but it only works in small ranges, making it ideal for holding atoms together.
What holds atoms together?
It is slack of force that holds atoms together. We call this the temperature of the substance. As temperature rises, the liquid state appears and the solid disappears. More temp and a Gas forms where aromsbtry to escape from one another,
What is the only force acting in chemistry at the level of atoms?
The only force acting in chemistry at the level of atoms is the electromagnetic force, basically +/- attraction. That’s it.
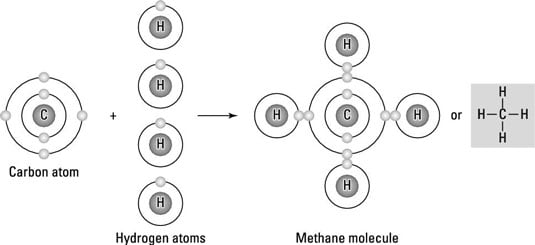
What happens when two hydrogen atoms are brought together?
Usually what happens when we bring two hydrogen atoms together, the repulsive force starts acting between the two nuclei as well as two electrons present. In addition to repulsive force an attractive force also starts acting between the electrons and nuclei. As you keep on bring the atoms together at equilibrium inter-nuclear distance the resultant force gets minimized and the molecule gets stabilized. At inter-nuclear distance the molecule has minimum energy
What is the charge of the nucleus?
The nuclei of each of the bonded atoms are positively charged. Each nucleus is attracted to a set of electrons which, after a fashion, sits between the two of them. The electrons are negatively charged, and are thus then held onto by both nuclei, keeping the nuclei in sync because of their mutual attraction to the same set of electrons. Think of it this way…. you and a friend are in a room with a front door and a back door. Your both pick up an end of a rope you see on the floor, and you are told not to let go of the rope no matter what. One of you is then instructed to leave by the front door
What forces hold the nucleus together?
Inside the nucleus are protons and neutrons. To get bigger than Hydrogen, you must have neutrons. They force positive protons (that want to move away from each other due to the EM force) to stay in the nucleus. Nuclear velcro is what I refer to the neutron as being. Protons and neutrons are massive. And beyond tiny. So tiny you can even think of them as taking up no space. Not much on the scale of the atom. This force that holds the nucleus together is the strongest force in the universe and thus we call it the strong force.
How are atoms held together?
So atoms are held together by the EM force (electron attracted to nucleus) and the nucleus of everything bigger than hydrogen depend on the strong force.
What are the two things that make up the nucleus?
Inside the nucleus are protons and neutrons. To get bigger than Hydrogen, you must