What is the homologous series of alcohol?
Alcohols are hydrocarbon compounds containing one or more hydroxyl group (O — H) attached to a saturated carbon atom. The alcohols which only contain one hydroxyl group attached to a saturated carbon form a homologous series. A hydrocarbon homologous series is a series of hydrocarbons which: Have the same general formula.
What is the homologous series of hydrocarbons?
A hydrocarbon homologous series is a series of hydrocarbons which: The general formula for the homologous series of alcohols is: where n is the number of carbon atoms and OH represents the hydroxyl group. Alcohols in the homologous series can be regarded as alkanes in which one hydrogen atom is replaced by a hydroxyl group.
What is the first member of the series for alcohol?
Also we know the general formula for alcohol which is C n H 2 n + 1 − O H . Here n is a positive integer value and it cannot be zero. Therefore the first member of the series will be C H 3 − O H , methyl-alcohol.
What is the fourth higher homologue of ethyl alcohol?
Also we know the general formula for alcohol which is C n H 2 n + 1 − O H . Here n is a positive integer value and it cannot be zero. Therefore the first member of the series will be C H 3 − O H , methyl-alcohol. ( i) When we are asking for a fourth higher homologue of ethyl alcohol it means we have to add four − C H 2 to the ethyl alcohol.

What is the general formula for alcohol?
The general formula for the alcohols is C nH 2n+1OH (where n is the number of carbon atoms in the molecule).
What is the formula of 3rd Member of alcohol homologous series?
The first three alcohols in the homologous series are methanol (CH3OH), ethanol (C2H5OH) and propanol(C3H7OH).
How do you find the general formula for a homologous series?
1:123:55A Level Chemistry Revision "General Formula" - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo the general formula for the alkanes is cn h2n plus two if we know the number of carbon atoms thenMoreSo the general formula for the alkanes is cn h2n plus two if we know the number of carbon atoms then we can use the general formula to work out the molecular formula for any alkane.
Which compound belongs to homologous series of alcohols?
Solution : Methanol `(CH_(3)OH)`, ethanol `(C_(2)H_(5)OH)` and propanol `(C_(3)H_(7)OH)` belong to the homologous series of alcohol since they all contain alcoholic functional group and have same characteristic properties.
What are the first 4 alcohols?
The four most common alcohols, which are also the simplest, are methanol (CH3OH), ethanol(C2H5OH), propanol (C3H7OH) and butanol (C4H9OH). The names of the alcohol are derived according to the alkane backbone.
What is the first member of alcohol?
MethanolMethanol CH3OH, also called methyl alcohol, wood alcohol, or wood spirit, the simplest of a long series of organic compounds called alcohols, consisting of a methyl group CH3 linked with a hydroxy group OH. Methanol was formally produced by the destructive distillation of wood.
What are the members of homologous series?
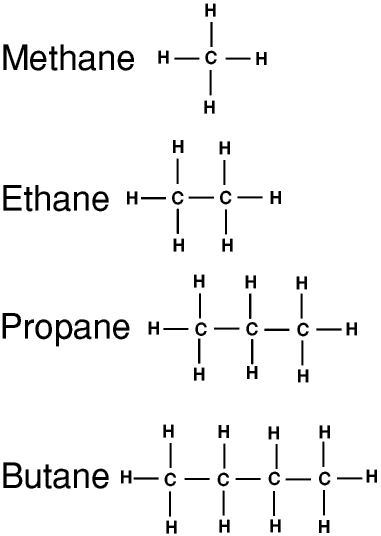
The homologous series of straight-chained alkanes begins methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), and pentane (C5H12).
Do homologous series have the same general formula?
A homologous series is a family of hydrocarbons with similar chemical properties who share the same general formula.
Which of the following is homologous series?
Solution : Butane, 2-methylbutane and 2, 3-dimethylbutane form a homologous series. All these hydrocarbons are alkanes.
How do you find your first member of alcohol?
-Alcohols are frequently commonly named as alcohol for the first four carbon atoms, which is the name of the alkyl group followed by alcohol. -According to IUPAC nomenclature, alcohols are named after the parent alkane name as –ol. Hence, Methanol $C{{H}_{3}}OH$is the first member of the alcohol series.
What is homologous series example?
Answer : A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group. For example, methane, ethane, propane, butane, etc. are all part of the alkane homologous series.
What is an alcohol functional group?
The functional group of an alcohol is the hydroxyl group, –OH. Unlike the alkyl halides, this group has two reactive covalent bonds, the C–O bond and the O–H bond. The electronegativity of oxygen is substantially greater than that of carbon and hydrogen.
What is the third member of alcohol?
propyl alcoholThe alcoholic functional group is hydroxyl (−OH). Thus, the first member of the alcohol homologous series is methyl alcohol, the second is ethyl alcohol, the third member is propyl alcohol and the fourth member is butyl alcohol.
What is the name and formula of 2nd member of homologous series of alcohols?
The second member of carbon compounds with group -OH (alcohol) is ethanol and its formula is CH3CH2OH.
What is homologous series write homologous series of alcohol?
A series of compounds in which the same functional group substitutes for hydrogen in the carbon chain is called homologous series . Consider the homologous series of alcohols- methanol, ethanol, etc. Note that methanol (CH3OH) is obtained by replacing one of the H atoms with -OH group in the methane structure.
Which is the third member of aldehyde series?
The third member of the homologous series of aliphatic aldehydes has the structure of propanal.
What is Homologous series?
Homologous series is a series of compounds with similar chemical properties and some functional group differing from the successive member by CH 2. Carbon chains of var ying length have been observed in organic compounds having the same general formula . Such organic compounds that vary from one another by a repeating unit and have the same general formula form a series of compounds. Alkanes with general formula C n H 2n+2, alkenes with general formula C n H 2n and alkynes with general formula C n H 2n-2 form the most basic homologous series in organic chemistry.
Why are the chemical properties of homologous series the same?
Chemical properties of the members of a homologous series are the same due to the fact that they all have the same functional groups in them. This series has enabled scientists and engineers to study different organic compounds systematically.
How do successive members differ from each other?
The successive members vary from each other by a CH 2 unit. For example in CH 4 and C 2 H 6, the difference is -CH 2 unit and the difference between C 2 H 6 and C 3 H 8 is also -CH 2 unit. So CH 4, C 2 H 6, and C 3 H 8 are homologs. The same thing can be observed in case of alkenes in which the first member is ethene and the successive members are C 3 H 6, C 4 H 8, and C 5 H 10. They differ from each other by a –CH 2 unit. Alkene formula is written as C n H 2n.
What properties depend on the mass and the total number of bonds in a compound?
Therefore, properties such as melting and boiling point, solubility, etc. that depend on the mass and the total number of bonds in a compound show a gradual change with an increase in molecular masses of the compounds.
What is the name of the group of alcohols in homologous series?
Alcohols in Homologous Series. called the hydroxyl group. The most common alcohol, known as ethanol, is used in alcoholic drinks, as fuel (gasoline), as a preservative for biological specimens, and as a solvent for paints and drugs. added to the parent chain of the alkane name.
What is a homologous series of organic compounds?
A homologous series is a series of organic compounds whose members can be denoted by a single general formula and differ from each other by a. – C H 2.
How to name aldehydes?
To name aldehydes, use the longest parent chain name of the alkane and replace ‘e’ of ‘ane’ with the suffix ‘-al’ . Thus, the name of C H 3 C H O is ethanal, and C H 3 C H 2 C H O is propanal. A homologous series for aldehydes is methanal, ethanal, propanal, and butanal with chemical formulas of H C H O, C H 3 C H O, C H 3 C H 2 C H O, and C H 3 C H 2 C H 2 C H O, respectively, where each successive compound differs from the previous one by a – C H 2 group.
What are some examples of homologous series?
Alkanes in Homologous Series. The simplest example of homologous series is the first four hydrocarbons: methane, ethane, propane, and butane. Methane having one carbon atom is surrounded by four hydrogen atoms. Hence, its molecular formula is.
What is the homologous series of alkenes?
Alkenes in Homologous Series. The homologous series of alkenes consists of ethene, propene, butene, pentene and so on. Ethene has two carbon atoms bonded to each other through a double bond and is surrounded by four hydrogen atoms. Hence, its molecular formula is.
What are the characteristics of homologous series?
What are the four characteristics of homologous series?#N#Ans: Four characteristics of a homologous series are:#N#(i) The general formula of all compounds in the series is the same.#N#(ii) They have the same functional group.#N#(iii) Their physical properties, such as melting point, boiling point, density, generally show a gradual change with an increase of molecular formula in the series.
What are the physical properties of homologous series?
1. The physical properties of members of a homologous series change gradually as the number of carbon atoms increases. 2. Each homologous series has its own functional group. These functional groups determine the chemical reactions that an organic molecule can undergo.
How to classify alcohols?
One way of classifying alcohols is based on which carbon atom is bonded to the hydroxyl group. If the carbon is primary ( 1 ∘, bonded to only one other carbon atom), the compound is a primary alcohol. Secondary alcohol has the hydroxyl group on a secondary (2 ∘) carbon atom, bonded to two other carbon atoms.
What is the name of the group of hydroxyl groups in alcohol?
Alcohols are the special class of organic compounds in which the hydrogen atom of an aliphatic carbon is replaced with the hydroxyl group (– OH). Thus, an alcohol molecule consists of two parts; one containing the alkyl group and the other containing the hydroxyl group. It is represented as R– OH, where R is the alkyl group.
What is Carboxylic Acid?
Carboxylic acids are the special class of organic compounds that incorporate a carboxyl functional group ( – {text {COOH}}.) The name carboxyl comes from the fact that a carbonyl group (left ( { – {text {C}} = {text {O}}} right)) and a hydroxyl group (left ( { – {text {OH}}} right)) are attached to the same carbon atom
How many pairs of electrons does the oxygen atom of the carbonyl group have?
The oxygen atom of the carbonyl group and that of the – O R group has two lone pairs each. The – O R oxygen allows one of its lone pair electrons to conjugate with the pi system of the carbonyl group. This makes the carboxyl group planar and can be represented with the following resonance structure.
What is the structure of ester?
Structure of Ester. To make an ester, a hydrogen atom from the hydroxyl group (– OH) of the alcohol and the – OH portion of the acid’s carboxyl group must be removed. The hydrogen atom and the – OH combine to form a water molecule (H2O). Propanol + Ethanoic acid ⇌ Propyl Ethanoate + water.
How many sigma bonds are there between carbonyl and hydroxyl?
The hydroxyl oxygen allows one of its lone pair electrons to conjugate with the pi system of the carbonyl group. The formation of 3 sigma bonds gives the carbonyl group a basic trigonal shape with bond angles of 120 degrees. The following diagram represents the resonance structure of an ester. The ester, in turn, balances between both structures. These two structures influence the stability and reactivity of an ester.
What is secondary alcohol?
Secondary alcohol has the hydroxyl group on a secondary (2 ∘) carbon atom, bonded to two other carbon atoms. Similarly, tertiary alcohol has the hydroxyl group on a tertiary (3 ∘) carbon atom, bonded to three other carbons. In all these types, the carbon atom and the oxygen atom is sp3 hybridised.
Process of Homologous Series
Alkanes in Homologous Series
Alkenes in Homologous Series
Alkynes in Homologous Series
Cycloalkane in Homologous Series
Functional Groups in Homologous Series
Alkyl Halides in Homologous Series
Alcohols in Homologous Series
Aldehydes and Ketones in Homologous Series
Carboxylic Acids in Homologous Series