The catalytic cycle helps to explain the mechanism of enzyme action through the following points:
- The substrate gets bound to the active site.
- This induces an alteration in the shape of the enzyme.
- The enzyme-product complex is formed by making and breaking bonds.
- Enzyme releases away from the product and it is available again for a fresh batch of substrates.
What are the 4 steps of enzyme action?
What are the steps of enzyme action?
- The enzyme and the substrate are in the same area. Some situations have more than one substrate molecule that the enzyme will change.
- The enzyme grabs on to the substrate at a special area called the active site.
- A process called catalysis happens.
- The enzyme releases the product.
What is the main function of enzymes?
These include:
- Enzymes help in signal transduction. ...
- They break down large molecules into smaller substances that can be easily absorbed by the body.
- They help in generating energy in the body. ...
- Enzymes are responsible for the movement of ions across the plasma membrane.
- Enzymes perform a number of biochemical reactions, including oxidation, reduction, hydrolysis, etc. ...
What factors affect enzyme activity?
- Enzymes are biological catalysts made up of large protein molecules. ...
- Enzymes are similar to other chemical catalysts. ...
- There are several factors that affect the speed of an enzyme’s action, such as the concentration of the enzyme, the concentration of the substrate, temperature, hydrogen ion concentration (pH), and ...
What are the three categories of enzymes?
What are the 3 types of enzymes?
- Amylase breaks down starches and carbohydrates into sugars.
- Protease breaks down proteins into amino acids.
- Lipase breaks down lipids, which are fats and oils, into glycerol and fatty acids.

What is the general mechanism of an enzyme MCQS?
The general mechanism is that an enzyme acts by: Reducing the activation energy.
What is the name for the mechanism of enzyme?
The catalytic cycle helps to explain the mechanism of enzyme action through the following points: The substrate gets bound to the active site. This induces an alteration in the shape of the enzyme. The enzyme-product complex is formed by making and breaking bonds.
What is the general structure of an enzyme?
Enzymes are proteins comprised of amino acids linked together in one or more polypeptide chains. This sequence of amino acids in a polypeptide chain is called the primary structure. This, in turn, determines the three-dimensional structure of the enzyme, including the shape of the active site.
What is enzyme classification and mechanism of action?
According to the International Union of Biochemists (I U B), enzymes are divided into six functional classes and are classified based on the type of reaction in which they are used to catalyze. The six kinds of enzymes are hydrolases, oxidoreductases, lyases, transferases, ligases and isomerases.
What are the two mechanism of enzyme action?
Mode of Enzyme Action: The mode of enzyme action depends upon the nature of the enzyme and the substrate molecule, and it can be understood by the following: (1) Formation of Enzyme Substrate complex (ESC): (2) Lowering of Activation energy.
What is the lock and key mechanism in enzymes?
Lock-and-key model is a model for enzyme-substrate interaction suggesting that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Enzymes are highly specific. They must bind to a specific substrate before they can catalyze a chemical reaction.
What is the general function of an enzyme in the body quizlet?
What is the function of an enzyme? They allow chemical reactions to occur at normal body temperature fast enough to sustain life. They reduce the activation energy needed to start a chemical reaction. You just studied 11 terms!
What is the general classification of enzymes?
Enzymes are classified into six categories according to the type of reaction catalyzed: Oxidoreductases, transferases, hydrolases, lyases, ligases, and isomerases.
What is the general function of an enzyme within a cell quizlet?
The function of enzymes in biological systems are to act as a catalyst to speed up chemical reactions in metabolism. Without the regulation by enzymes, chemical traffic trough the pathways of metabolism would become terribly congested because many chemical reactions would take such a long time.
What is the mechanism of enzyme action GCSE?
Step One: Enzymes and substrates randomly move about in solution. Step Two: When an enzyme and its complementary substrate randomly collide, an enzyme-substrate complex forms and the reaction occurs. Step Three: A product (or products) forms (from the substrate) and is then released from the active site.
What are the 4 steps for enzyme action?
Four Steps of Enzyme ActionThe enzyme and the substrate are in the same area. Some situations have more than one substrate molecule that the enzyme will change.The enzyme grabs on to the substrate at a special area called the active site. ... A process called catalysis happens. ... The enzyme releases the product.
What are the different factors that affect on mechanism of enzyme action?
Enzyme activity can be affected by a variety of factors, such as temperature, pH, and concentration. Enzymes work best within specific temperature and pH ranges, and sub-optimal conditions can cause an enzyme to lose its ability to bind to a substrate.
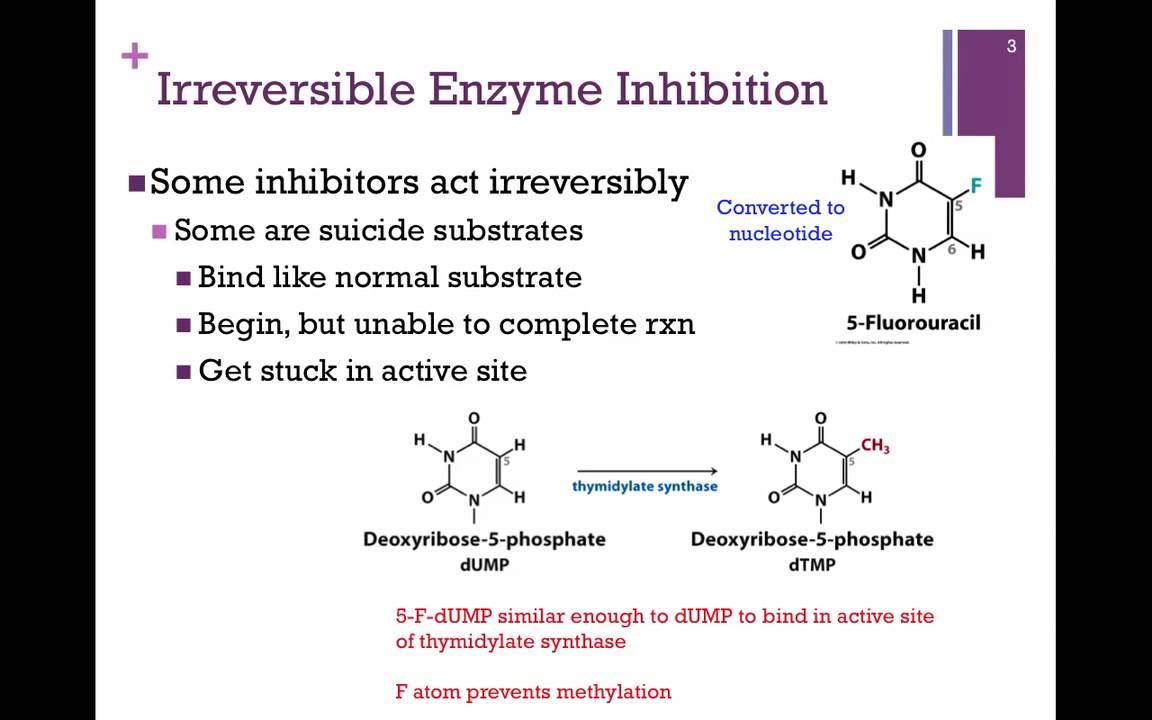
What is the name of enzyme inhibition?
A decrease in enzyme-related processes, enzyme production, or enzyme activity is referred to as enzyme inhibition. Competitive, Non-competitive, and Uncompetitive are the three types of inhibition reactions.
What is another name for enzymes?
Enzymes Synonyms - WordHippo Thesaurus....What is another word for enzymes?fermentsyeastbacteriamouldsUKmoldUSraising agentsfermentleavenleaveningenzyme4 more rows
What is the mechanism of enzyme action GCSE?
Step One: Enzymes and substrates randomly move about in solution. Step Two: When an enzyme and its complementary substrate randomly collide, an enzyme-substrate complex forms and the reaction occurs. Step Three: A product (or products) forms (from the substrate) and is then released from the active site.
What is an enzyme?
An enzyme is a substance that acts as a catalyst in living organisms, regulating the rate at which chemical reactions proceed without itself being...
What are enzymes composed of?
A large protein enzyme molecule is composed of one or more amino acid chains called polypeptide chains. The amino acid sequence determines the char...
What are examples of enzymes?
Practically all of the numerous and complex biochemical reactions that take place in animals, plants, and microorganisms are regulated by enzymes,...
What factors affect enzyme activity?
Enzyme activity is affected by various factors, including substrate concentration and the presence of inhibiting molecules. The rate of an enzymati...
What is the role of enzymes in a catalyzed reaction?
Enzymes lower the activation energy or increase the reaction rate (conversion of substrate into a product).
What is substrate in chemistry?
Substrates: In terms of enzymology, the substrates refers to the reactants molecules, which form a temporary association with an enzyme or turn out to form an enzyme-sub strate complex ( E-S complex ). Various bonds form between the initial contact of the two, i.e. an enzyme and substrate that releases binding energy to create a perfect fit.
What are the steps of enzyme catalysis?
Enzyme catalysis is necessary for many biological or biochemical pathways to occur or essential for the chemical interconversions that sustain life. Let us look into a few examples of enzyme catalysis: 1 Sucrose (disaccharide) converts into two different monosaccharide molecules, i.e. glucose and fructose, via the enzyme action of “ Sucrase ”. 2 Glucose (monosaccharide) converts into ethanol (primary alcohol) and atmospheric carbon dioxide via the action of the enzyme “ Zymase ”.
How many times can an enzyme catalyze the same chemical pathway?
Enzymes can catalyze the same chemical pathway several times until they get denatured and associate with the inhibitors. In this context, we will study the mechanism of enzyme action through three popular models (lock and key hypothesis, Induced fit model and Michaelis and Menten’s equation. You will also get to know the difference between ...
Why are enzymes specific?
Enzymes are specific due to the presence of a distinct region called an active site of an enzyme. Enzyme action: It is defined as the enzyme’s activity, which facilitates the catalysis or breakdown of chemical substrates (participating in the reaction) into the desired products. Therefore, the term “Enzyme action” is sometimes interchangeable ...
Why does a substrate enter the transition state?
In a catalyzed reaction or an enzyme’s presence, the substrate rapidly reaches the transition state due to decreased activation energy. The enzyme reduces the energy required (activation energy) for the substrate to form products. Conversely, the substrates take more time to reach the transition state and form products without an enzyme catalyst.
What is the transition state of enzymes?
Therefore, to study the enzyme’s mechanism more in detail, we must know the meaning of the following terms: Transition state: It refers to the high energy state during which the substrates are in the process of falling into the products. The transition state is the intermediary stage between the substrate and product, which remains unstable, ...
What are the advantages of enzymes in a catalyst?
Enzyme-catalyzed reactions offer enhanced specificity and selectivity over traditional catalysts, but in the native form, many enzymes suffer poor solvent stability and thermostability. To improve their performance and ease of recovery in industrial applications, enzymes have been immobilized on solid supports ( Talbert & Goddard, 2012 ). Immobilization has been achieved through numerous methods including covalent attachment, physical adsorption, cross-linking, self-assembly, and entrapment ( Hwang & Gu, 2013; Sheldon & Pelt, 2013 ). Physical adsorption achieves high-protein loading on a surface with minimal activity losses. The main drawback to physical adsorption is the leaching of enzyme from the solid support, thus leading to decreased activity over multiple uses ( Bastida et al., 1998; Jun et al., 2013; Wiemann et al., 2009 ). Enzymes can be covalently bound to solid supports or to other enzymes to form cross-linked aggregates ( Sheldon, 2011) and covalent attachment prevents leaching of the enzyme during the catalytic cycles, but cross-linkers can alter enzyme conformation, and the chemistry used for linking may damage the delicate active site residues and thus reduce enzymatic activity of the resulting biocatalyst ( Arroyo, Sánchez-Montero, & Sinisterra, 1999; Poppe, Costa, Brasil, Rodrigues, & Ayub, 2013 ). Entrapment or encapsulation of enzymes in inert matrices can facilitate the incorporation of enzymes into larger materials. The core material used to entrap the enzyme may inhibit substrate interaction with the active site of the enzyme, or enzymes may escape the matrix if the pores of the encapsulating matrix are too big ( Yagonia, Park, & Yoo, 2013 ). Nanomaterials offer increased surface area to volume ratio, enabling increased protein loading and reduced protein–protein and protein–surface interactions that may result in activity loss ( Jeong, Duncan, Park, Kim, & Rotello, 2011; Talbert et al., 2014 ). However, practical challenges such as recovery of the nanoparticle and their toxicity concerns have limited commercial adoption of biocatalytic nanomaterials in some industries.
How is rubber biosynthetic?
Rubber biosynthetic activity is usually quantified by the incorporation of radiolabeled IPP into rubber by enzymatically active latex or purified rubber particles. This works well because the newly synthesized rubber can be detected in the background of previously synthesized unlabeled rubber. However, kinetics can only usefully be examined in the purified rubber particle system. In latex, the IPP isomerase will convert 14 C-IPP into 14 C-DMAPP, and then the trans prenyl transferases can make 14 C-labeled FPP with label coming from the initiator and the IPP monomer in unknown proportions. Even if the isomerase is inhibited, the prenyl transferases can still incorporate the 14 C-IPP into FPP, which has a considerably higher binding affinity for the rubber transferase active site than the smaller APPs. Incorrect control of competing enzymes has led to erroneous interpretation of rubber biosynthetic assay results. For example, a stereochemical shift of FPP synthase (FPS) from a C15 trans prenyl transferase enzyme to a high-molecular-weight cis prenyl transferase, via recombination of FPS with a sole membrane-bound 14.6 kDa protein, was deduced ( Dennis & Light, 1989; Light & Dennis, 1989; Light, Lazarus, & Dennis, 1989 ). It was subsequently shown, however, that the trans prenyl transferase preparation used also contained IPP isomerase ( Cornish, 1993 ), and the Coomassie stain used to indicate the presence of a single rubber particle-bound protein failed to detect many other bound proteins. See Section 5 for a comparison of Coomassie- and silver-stained gels.
Why is the core material in the above design selected?
Furthermore, the core material in the above design can be selected to enable better performance even in nonaqueous solvent systems which might be more suitable for certain hydrophobic substrates or when water is not desirable in the reaction medium. This rational design of hierarchical interfacial assembly of the biocatalyst at multiple levels is a major advance in controlling the biocatalyst properties at the molecular level. It is also a facile method to create reusable biocatalysts with dramatically improved stability against temperature, pH, and solvent-induced denaturation, as demonstrated here.
How do bioelectrocatalytic systems work?
In order to control these processes by applying external magnetic field some components of the bioelectrocatalytic systems can be conjugated with nano- or micro-size magnetic particles. These may include covalent binding of redox enzymes to magnetic particles or, in the easier approach, either electron transfer mediators or cofactors can be associated with magnetic particles. The magneto-induced translocation of the functionalized magnetic particles to and from an electrode surface will result in the activation and inhibition of bioelectrocatalytic processes, respectively. This approach to the magneto-switchable bioelectrocatalytic reactions has been realized with many different magnetic particle conjugates moving them to and from electrodes ( Katz, 2016 ).
What are uni-reactions?
Enzyme reactions with a single substrate and a single product, such as those described above, are called uni-reactions and represent a minority of the reactions that occur in metabolism Palmer (1995b). For multisubstrate reactions, reactants are designated A, B, C, D… for the uni-, bi-, ter-, and quad-reactant substrates. The products are designated P, Q, R, S…. The Michaelis-Menten equations are similar to that considered earlier, but the complexity increases considerably Voet et al (1999).
How to determine the pH of rubber transferases?
The pH optima of rubber transferases can be determined using universal buffering systems, and the acidic and basic thresholds for denaturation, in contrast to inhibition, determined.
What are the two main conformations of Na,K-ATPase?
E1 and E2 indicate the two main conformation of the Na,K-ATPase. In E1, the cation-binding sites are accessible from the intracellular side of the membrane and in E2 from the extracellular side. The parentheses indicate that the cations are occluded in the protein. The o and i indices denote the extracellular (o) and intracellular (i) side of the membrane. One phosphate (P) is transferred from ATP to the protein (E2∼P) and then released as inorganic phosphate (Pi). ATP binds with a low-affinity site (∼100 μM, a) to the E2 (K 2) form promoting the conversion to the E1 form and the release of K. In the presence of low-ATP concentration, an alternate slower reaction path is followed (shown in gray) in which K is released first and then ATP binds to a high-affinity site (∼1 μM, b). The affinities indicated in the text for the cations are those for the intracellular-binding site on E1 for Na (c) and the extracellular site on E2 for K (d). The curved arrow in the center indicates the direction of the reaction under physiological conditions.
How is the rate of an enzymatic reaction determined?
Thus, enzymatic reaction rate is determined by the speed at which the active sites convert substrate to product.
How does competitive inhibition occur?
Competitive inhibition occurs when molecules similar to the substrate molecules bind to the active site and prevent binding of the actual substrate.
How are enzymes named?
Because of this specificity, enzymes often have been named by adding the suffix “-ase” to the substrate’s name (as in urease, which catalyzes the breakdown of urea ). Not all enzymes have been named in this manner, however, and to ease the confusion surrounding enzyme nomenclature, a classification system has been developed based on the type of reaction the enzyme catalyzes. There are six principal categories and their reactions: (1) oxidoreductases, which are involved in electron transfer; (2) transferases, which transfer a chemical group from one substance to another; (3) hydrolases, which cleave the substrate by uptake of a water molecule (hydrolysis); (4) lyases, which form double bonds by adding or removing a chemical group; (5) isomerases, which transfer a group within a molecule to form an isomer; and (6) ligases, or synthetases, which couple the formation of various chemical bonds to the breakdown of a pyrophosphate bond in adenosine triphosphate or a similar nucleotide.
What are the two things that enzymes do?
Enzymes catalyze all aspects of cell metabolism. This includes the digestion of food, in which large nutrient molecules (such as proteins, carbohydrates, and fats) are broken down into smaller molecules; the conservation and transformation of chemical energy; and the construction of cellular macromolecules from smaller precursors.
Why is an energy barrier necessary in chemical reactions?
This barrier prevents complex molecules such as proteins and nucleic acids from spontaneously degrading, and so is necessary for the preservation of life.
What is the role of enzymes in a chemical reaction?
Enzyme, a substance that acts as a catalyst in living organisms, regulating the rate at which chemical reactions proceed without itself being altered in the process. In the induced-fit theory of enzyme-substrate binding, a substrate approaches the surface of an enzyme (step 1 in box A, B, C) and causes a change in the enzyme shape ...
What is the active site of an enzyme?
The active site of an enzyme is a groove or pocket that binds a specific substrate. Encyclopædia Britannica, Inc. Enzyme synthesis and activity also are influenced by genetic control and distribution in a cell. Some enzymes are not produced by certain cells, and others are formed only when required.
How Do Enzymes Work?
Enzymes speed up chemical reactions in the body, making things go faster than they normally would. But how do they accomplish this feat? Well, every reaction has an initial barrier called activation energy. Activation energy is like the hump the reaction has to get over before it can get started. Even reactions that net a production of energy still need to break this barrier. Think of it like pushing a car that broke down. It's really hard to get started, but once you and your friends get some momentum going, the car starts to roll and you can ease it to the side of the road.
How does an enzyme affect the reaction?
When the enzyme binds the substrate, it holds it in a way that orients it for the reaction. For example, if two molecules are being attached by the enzyme, the enzyme holds them in a way that the sites that should be connected are easily accessible. Without the enzyme, the reactants would randomly have to land together this way, which is unlikely. Although enzymes speed up the reactions, they don't change them. The end result is still the same, whether you use an enzyme or not. The only difference is how fast it gets done.
What enzymes use metals to speed up reactions?
We'll just look at one example where enzymes use metals to help speed up reactions. Carbonic anhydrase is an enzyme that uses metal ion catalysis. It converts carbon dioxide and water into bicarbonate and hydrogen ions, and can also catalyze the reverse reaction when needed.
What is the zinc ion in carbonic anhydrase?
Inside the active site, or the location where the substrate binds, carbonic anhydrase has a zinc ion. The zinc ion inside the active site takes a hydrogen atom from water, allowing water to become negative. The negative water ion, called a hydroxide group, attacks carbon dioxide.
How does the substrate affect the enzyme?
Once attached, the substrate causes an induced fit in the enzyme, where the enzyme changes shape so it can fit even better. The enzyme orients the reactants in a better way towards each other, and uses molecules like metals to speed up the reaction. To unlock this lesson you must be a Study.com Member. Create your account.
How do enzymes make a reaction go faster?
Enzymes lower the activation energy of a reaction, which helps it go faster. Some enzymes, like carbonic anhydrase, which converts carbon dioxide to bicarbonate in the blood, make the reaction proceed nearly a million times faster than without the enzyme just by lowering activation energy.
How does enzyme speed up chemical reactions?
An enzyme is a protein catalyst that speeds up chemical reactions. It does this by decreasing the activation energy of a reaction. Reactions can be sped up over a million times by enzymes like carbonic anhydrase, which uses zinc ions to convert carbon dioxide and water to hydrogen ions and bicarbonate.
What is the mechanism of enzyme action?
Mechanism of Enzyme Action: Enzyme is active in catalytic action of biochemical reaction. They act on substrate and forms a complex after interactions with the enzyme is called active center. The enzyme and substrate forms a complex at the active centre. This binding action makes both enzyme and substrate stable.
Why is the key of an enzyme the substrate?
Since product has lower free energy, it is released. Enzymes are fixed to receive another molecule of substrate and thus enzyme activity continues. In this analogy, the lock is the substrate and the key is the enzyme. Only the correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
How much does an enzyme weigh?
Enzymes are the large globular proteins with molecular weight ranging from 13,000 to millions Dalton. The catalytic efficiency of an enzyme depends upon its three dimensional conformation.
Where can a substrate be found?
According to the particular substrate can be found at active site of particular enzyme forming substrate-enzyme complex . Enzyme-substrate complex remains in tight fitting and active sites of enzymes are complementary to substrate molecules.
What is enzyme in biology?
Enzymes are highly specialized proteins which act as catalyst of biological system . Louis Pasteur was the first to recognize the importance of enzymes while studying the fermentation process and denoted it as “ferment”-an integral part of living cells.
Why are cysteine residues more stable?
Enzymes with more cysteine residues are thermally more stable because of the formation of -S-S bridges between the peptide chains.
How many classes are there in the enzymatic system?
This system has been recommended by International Committee for Nomenclature of Enzymes in 1973. This system includes six major classes including other subclasses according to the type of reaction catalyzed (Table 12.2).
How does the catalytic cycle work?
By breaking and making the bonds, the substrate binds to the enzyme (remains unchanged), which converts into the product and later splits into product and enzyme. The free enzymes then bind to other substrates and the catalytic cycle continues until the reaction completes.
Why is the regulation of enzymes important?
The regulation of enzymes has been a key element in clinical diagnosis because of their role in maintaining life processes. The macromolecular components of all enzymes consist of protein, except in the class of RNA catalysts called ribozymes. The word ribozyme is derived from the ribonucleic acid enzyme. Many ribozymes are molecules of ribonucleic acid, which catalyze reactions in one of their own bonds or among other RNAs.
How many amino acids are in an enzyme?
Compared to its substrates, enzymes are typically large with varying sizes, ranging from 62 amino acid residues to an average of 2500 residues found in fatty acid synthase. Only a small section of the structure is involved in catalysis and is situated next to the binding sites.
What are the functions of enzymes?
The enzymes perform a number of functions in our bodies. These include: 1 Enzymes help in signal transduction. The most common enzyme used in the process includes protein kinase that catalyzes the phosphorylation of proteins. 2 They break down large molecules into smaller substances that can be easily absorbed by the body. 3 They help in generating energy in the body. ATP synthase is the enzymes involved in the synthesis of energy. 4 Enzymes are responsible for the movement of ions across the plasma membrane. 5 Enzymes perform a number of biochemical reactions, including oxidation, reduction, hydrolysis, etc. to eliminate the non-nutritive substances from the body. 6 They function to reorganize the internal structure of the cell to regulate cellular activities.
What is a fad in enzymes?
Prosthetic groups: These are cofactors tightly bound to an enzyme at all times. A fad is a prosthetic group present in many enzymes .
What is the name of the enzyme that catalyzes the structural shifts present in a molecule?
Adds water, carbon dioxide or ammonia across double bonds or eliminate these to create double bonds. Isomerases. The Isomerases enzymes catalyze the structural shifts present in a molecule, thus causing the change in the shape of the molecule. Ligases.
What is the structure of an enzyme?
Enzymes are a linear chain of amino acids, which give rise to a three-dimensional structure . The sequence of amino acids specifies the structure, which in turn identifies the catalytic activity of the enzyme. Upon heating, enzyme’s structure denatures, resulting in a loss of enzyme activity, that typically is associated with temperature.
How many isoforms of AK are there?
To date there have been nine human AK protein isoforms identified. While some of these are ubiquitous throughout the body, some are localized into specific tissues. For example, AK7 and AK8 are both only found in the cytosol of cells; and AK7 is found in skeletal muscle whereas AK8 is not. Not only do the locations of the various isoforms within the cell vary, but the binding of substrate to the enzyme and kinetics of the phosphoryl transfer are different as well. AK1, the most abundant cytosolic AK isozyme, has a Km about a thousand times higher than the Km of AK7 and 8, indicating a much weaker binding of AK1 to AMP. Sub-cellular localization of the AK enzymes is done by unique targeting sequences found in the protein. Each isoform also has different preference for NTP’s. Some will only use ATP, whereas others will accept GTP, UTP, and CTP as the phosphoryl carrier.
What type of restriction enzymes are found in bacteria?
As with other classes of restriction enzymes, Type II Restriction Enzymes occur exclusively in unicellular microbial life forms––mainly bacteria and archaea (prokaryotes)––and are thought to function primarily to protect these cells from viruses and other infectious DNA molecules. Inside a prokaryote, the restriction enzymes selectively cut up foreign DNA in a process called restriction digestion; meanwhile, host DNA is protected by a modification enzyme (a methyltransferase) that modifies the prokaryotic DNA and blocks cleavage. Together, these two processes form the restriction modification system.
What is the name of the enzyme that cuts DNA?
A restriction enzyme, restriction endonuclease , or restrictase is an enzyme that cleaves DNA into fragments at or near specific recognition sites within molecules known as restriction sites. Restriction enzymes are one class of the broader endonuclease group of enzymes. Restriction enzymes are commonly classified into five types, which differ in their structure and whether they cut their DNA substrate at their recognition site, or if the recognition and cleavage sites are separate from one another. To cut DNA, all restriction enzymes make two incisions, once through each sugar-phosphate backbone (i.e. each strand) of the DNA double helix. Here we will focus on the Type II restriction enzymes that are routinely used in molecular biology and biotechnology applications.
What is the role of adenylate kinase in ATP?
Adenylate kinase (also known as AK or myokinase) is a phosphotransferase enzyme that catalyzes the interconversion of adenine nucleotides (ATP, ADP, and AMP). By constantly monitoring phosphate nucleotide levels inside the cell, AK enzymes play an important role in cellular energy homeostasis. The basic chemical reaction mediated by this enzyme class is the conversion of 2 ADP molecules into 1 ATP and 1 AMP (Figure 7.20A). The reverse reaction can also occur forming an equilibrium based on cellular concentrations of the varying phosphorylation states.
What is the name of the enzyme that cleaves peptide bonds?
Proteolytic enzymes belong to the hydrolase class of enzymes and are grouped into the subclass of the peptide hydrolases or peptidases. Depending on the site of enzyme action the proteases can also be subdivided into exopeptidases or endopeptidases. Exopeptidases, such as aminopeptidases and carboxypeptidases catalyze the hydrolysis of the peptide bonds near the N– or C-terminal ends of the substrate, respectively (Figure 7.16). Endopeptidases (Figure 7.16) cleave peptide bonds at internal locations within the peptide sequence. Proteases may also be nonspecific and cleave all peptide bonds equally or they may be highly sequence specific and only cleave peptides after certain residues or within specific localized sequences.
What is the heme cofactor?
The family of heme cofactors contain an iron metal coordinated with a porphyrin ring structure as shown in the left hand panel within the structure of Heme B. In the right hand panel, Heme B is shown complexed with the succinate dehydrogenase enzyme from the Kreb Cycle.
What are the two cofactors of enzymes?
They can be divided into two major categories: metals and coenzymes . Metal cofactors that are commonly found in human enzymes include: iron, magnesium, manganese, cobalt, copper, zinc, and molybdenum. Coenzymes are small organic molecules that are often derived from vitamins, which are essential organic nutrients consumed within the diet. Coenzymes can bind loosely with the enzyme and have the ability to bind and release from the active site, or they may be tight binding and lack the ability to release easily from the enzyme. Tight binding coenzymes are referred to as prosthetic groups. Enzymes that are not yet associated with a required cofactor are called apoenzymes, whereas enzymes that are bound with their required cofactors are called holoenzymes. Sometimes organic molecules and metals combine to form coenzymes, such as in the case of the heme cofactor (Figure 7.15). Coordination of heme cofactors with their enzyme counterparts often involves electrostatic interactions with histidine residues as shown in the succinate dehydrogenase enzyme shown in Figure 7.15.
Content: Mechanism of Enzyme Action
Important Terms
- Before proceeding to the mechanism of enzyme action, we must have a brief knowledge of the following terms: Enzymes: These are the 3-D proteinaceous organic compounds, which function as a “biocatalyst” to increase the reaction’s speed. Enzymes are specific due to the presence of a distinct region called an active site of an enzyme. Enzyme action: It is defined as the enzyme’s a…
Mechanism
- The mechanism of enzyme action typically depends upon the activation energy. Enzymes participating in any chemical reaction reduce the activation energy and decreasing the time between the substrate’s interconversion into a product. Therefore, to study the enzyme’s mechanism more in detail, we must know the meaning of the following terms: Transitio...
Lock and Key Hypothesis
- It was pioneered by a scientist named Emil Fischer(in 1894), which explains the enzyme’s mechanism. According to this model, an active site is a region of the enzyme, which bears a specific shape or conformation. Lock and key hypothesis have a simple approach, which says that the particular substrate perfectly fits into the enzyme’s cleft (active site) for the reaction to occur. …
Induced Fit Model
- It is the widely accepted model to study the mechanism of enzyme action and pioneered by the scientist Daniel Koshland (in 1959). According to his theory, an active site is a flexible region of the enzyme, which can undergo conformational changes. It is also popular by the name of the hand in glove model. The induced-fit model explains that the enzyme’s active site possesses tw…
Michaelis and Menten’s Model
- Leonor Michaelis and Maud Menten gave an equation in 1913 to explain the mechanism of enzyme action. It depends upon the lowering of activation energy. According to Michaelis Menten’s equation, the enzyme-substrate complex can reversibly dissociate into (enzyme plus substrate) and further proceed to give (enzyme plus product). In a catalyzed reaction or an enzy…