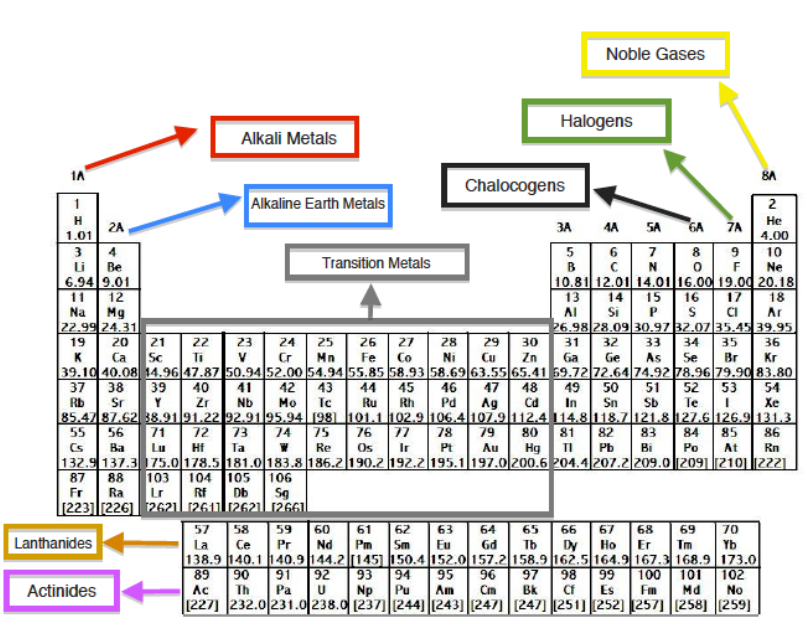
Group
- Group 1: alkali metals, or lithium family
- Group 2: alkaline earth metals, or beryllium family
- Group 3: the scandium family
- Group 4: the titanium family
- Group 5: the vanadium family
- Group 6: the chromium family
- Group 7: the manganese family
- Group 8: the iron family
- Group 9: the cobalt family
- Group 10: the nickel family
What are the six groups in the periodic table?
Jan 23, 2020 · Beside this, what are the group names of the periodic table? The following names for specific groups in the periodic table are in common use: Group 1: alkali metals. Group 2: alkaline earth metals. Group 11: coinage metals (not an IUPAC approved name) Group 15: pnictogens (not an IUPAC approved name) Group 16: chalcogens. Group 17: halogens. Group …
What is group 17 period 5 on the periodic table?
Jul 08, 2021 · Group 5A — The Pnictogens. Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi). What are the groups on the periodic table called? Periodic Table of Elements. The periodic table is the tabular arrangement of all the chemical …
What is group 5a on periodic table?
Nov 30, 2021 · Group 5 (by IUPAC style) is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. The group itself has not acquired a trivial name; it belongs to the broader grouping of the transition metals.
What do the groups of the periodic table tell us?
Feb 28, 2022 · By. famousfaqs. Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids …
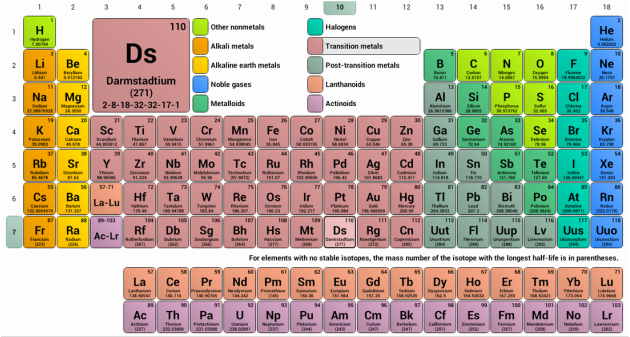
What is element 5 in the periodic table?
BoronBoron - Element information, properties and uses | Periodic Table.
Is group 5 on the periodic table metals?
1, the trends in properties of the group 5 metals are similar to those of group 4. Only vanadium, the lightest element, has any tendency to form compounds in oxidation states lower than +5....Group 5 Elemental Properties.ElementTaElectronegativity1.50Metallic Radius (pm)146Melting Point (°C)3017Density (g/cm3)16.654 more columns•Aug 15, 2020
What is the name of Group 6 on the periodic table?
Group 6, numbered by IUPAC style, is a group of elements in the periodic table. Its members are chromium, molybdenum, tungsten, and seaborgium. These are all transition metals and chromium, molybdenum and tungsten are refractory metals.
Why are group 5 elements called pnictogens?
Also Known As: Elements belonging to this group are also known as pnictogens, at term derived from the Greek word pnigein, which means "to choke". This refers to the choking property of nitrogen gas (as opposed to air, which contains oxygen as well as nitrogen).May 7, 2019
What are the 5 metalloids?
The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium, and astatine.
What element is in Group 5 Period 5?
The period 5 transition metals are yttrium (Y), zirconium (Zr), niobium (Nb), molybdenum (Mo), technetium (Tc), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), and cadmium (Cd).
What is Group 4 called in the periodic table?
the titanium groupGroup 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lightest member.
What is the name of Group 7 in periodic table?
Group 7 Elements Also called the halogens.
What is Group 7 called?
the halogensThe Group 7 elements are called the halogens. They are placed in the vertical column, second from the right, in the periodic table . Chlorine, bromine and iodine are the three common Group 7 elements.
What is aluminum's family name?
Boron FamilyBoron Family This group includes the elements boron, aluminum, gallium, indium, and thallium.
What is the meaning of pnictogens?
A pnictogen (/ˈpnɪktədʒən/ or /ˈnɪktədʒən/; from Ancient Greek: πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. This group is also known as the nitrogen family.
Which group 5A element is most metallic?
Bismuth is the only metal in the group and it is the heaviest element that contains stable (non-radioactive) isotopes. Elements of Group 5A overwhelmingly form covalent compounds.
What Is Group 5a On The Periodic Table?
Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi). Group 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb
Where is group 5A on the periodic table?
Let’s quickly look at the group 5A elements, which are located towards the right side of the periodic table. Group 5A includes Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi). Sometimes group 5A is known as Group 15 or Group VA, it just depends on the periodic table you are viewing.
What does group 5 mean in the periodic table?
Group 5 (by IUPAC style) is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. The group itself has not acquired a trivial name; it belongs to the broader grouping of the transition metals.
What element is in Group 5 Period 5?
Rubidium is the first element placed in period 5. It is an alkali metal, the most reactive group in the periodic table, having properties and similarities with both other alkali metals and other period 5 elements.
Which group 5A element is the most metallic?
Bismuth is the only metal in the group and it is the heaviest element that contains stable (non-radioactive) isotopes. Elements of Group 5A overwhelmingly form covalent compounds.
Which 5A element is most metallic?
The most metallic element is francium. However, francium is a man-made element, except for one isotope, and all isotopes are so radioactive they almost instantly decay into another element. The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table.
What ionic charge does Group 5A have?
That is, the Group 7A nonmetals form 1- charges, the Group 6A nonmetals form 2- charges, and the Group 5A metals form 3- charges.