
The ionic radius (plural: ionic radii) is the measure of an atom
Atom
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…
What ion has the smallest radius?
Out of potassium and calcium ion, calcium ion will have the smallest radius due to the removal of two electrons. Which one of the ion has smallest radius Nks CA?
Which ion would have a smaller radius?
Cations, which have lost at least 1 electron, carry a positive charge and their ionic radius is smaller due to this charge imbalance. Anions, on the other hand, have gained one or more electrons and have a net negative charge; anions have a larger radius due to these excess electrons. Just so, why do positive ions have a smaller radius?
How to calculate the radius of an ion?
- Find the radii of anion (r -) atom.
- Find internuclear distance (d) between anion and cation.
- Use Internuclear distance formula to find the r +.
Which ions has the smallest atomic radius?
Thus, helium is the smallest element, and francium is the largest. Which ion has the smallest radius quizlet? Ca2+ would have the smallest ionic radius because Calcium has a positive charge, and because this ion is a cation, cations will have the smallest radius. So therefore, elements with a positive charge, will have a smaller ionic radius.
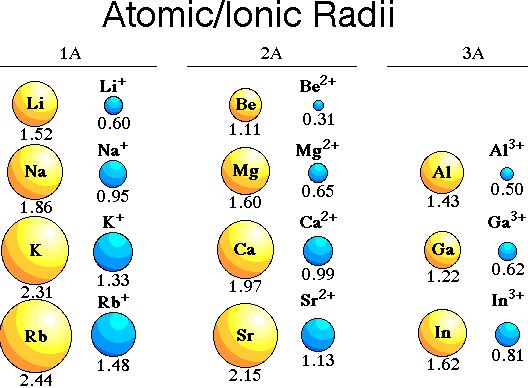
What is the ionic radius of an ion?
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are sometimes treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).
What is an anomalous ionic radius?
An "anomalous" ionic radius in a crystal is often a sign of significant covalent character in the bonding. No bond is completely ionic, and some supposedly "ionic" compounds, especially of the transition metals, are particularly covalent in character. This is illustrated by the unit cell parameters for sodium and silver halides in the table. On the basis of the fluorides, one would say that Ag + is larger than Na +, but on the basis of the chlorides and bromides the opposite appears to be true. This is because the greater covalent character of the bonds in AgCl and AgBr reduces the bond length and hence the apparent ionic radius of Ag +, an effect which is not present in the halides of the more electropositive sodium, nor in silver fluoride in which the fluoride ion is relatively unpolarizable .
How did Wasastjerna find the ionic radii?
Wasastjerna estimated ionic radii by considering the relative volumes of ions as determined from electrical polarizability as determined by measurements of refractive index. These results were extended by Victor Goldschmidt. Both Wasastjerna and Goldschmidt used a value of 132 pm for the O 2− ion.
What are Shannon's ionic radii?
A major review of crystallographic data led to the publication of revised ionic radii by Shannon. Shannon gives different radii for different coordination numbers, and for high and low spin states of the ions. To be consistent with Pauling's radii, Shannon has used a value of rion (O 2− ) = 140 pm; data using that value are referred to as "effective" ionic radii. However, Shannon also includes data based on rion (O 2− ) = 126 pm; data using that value are referred to as "crystal" ionic radii. Shannon states that "it is felt that crystal radii correspond more closely to the physical size of ions in a solid." The two sets of data are listed in the two tables below.
How to find ionic radii?
Landé estimated ionic radii by considering crystals in which the anion and cation have a large difference in size, such as LiI. The lithium ions are so much smaller than the iodide ions that the lithium fits into holes within the crystal lattice, allowing the iodide ions to touch. That is, the distance between two neighboring iodides in the crystal is assumed to be twice the radius of the iodide ion, which was deduced to be 214 pm. This value can be used to determine other radii. For example, the inter-ionic distance in RbI is 356 pm, giving 142 pm for the ionic radius of Rb +. In this way values for the radii of 8 ions were determined.
How to determine the distance between two ions in an ionic crystal?
The distance between two ions in an ionic crystal can be determined by X-ray crystallography, which gives the lengths of the sides of the unit cell of a crystal. For example, the length of each edge of the unit cell of sodium chloride is found to be 564.02 pm.
How does an added electron affect the size of an atom?
Similarly, when an electron is added to an atom, forming an anion, the added electron increases the size of the electron cloud by interelectronic repulsion. The ionic radius is not a fixed property of a given ion, but varies with coordination number, spin state and other parameters. Nevertheless, ionic radius values are sufficiently transferable ...
What is the ionic radius of an atom?
Ionic radius is the radius of an atom’s ion. Ions cannot exist alone. If it is a positively charged ion, it will react with a negatively charged ion (or the opposite) and become a stable neutral compound. This compound is called an ionic compound because it is made out of ionic components.
How to calculate ionic radius?
Ionic Radius: Ionic radius can be calculated by dividing the distance between two nuclei of two ions according to their sizes.
How to find the ionic radius of an ionic compound?
The most accurate way to find the ionic radius is to divide the distance between two nuclei of two ions according to their sizes. For example, if an ionic compound is composed of a cation and an anion that has an atomic size which is three times larger, the distance between the two nuclei should be divided by 4 in order to obtain the cation radius.
What happens to the atomic radius when the number of electrons is higher?
If the number of electrons present is higher, the attraction between the electrons and the nucleus is also high. In the beginning of the period, there is a less number of electrons present in the outermost orbital. Then the attraction from the nucleus is less. Therefore, the atom is large, and the atomic radius is also large.
How does the atomic radius change?
The elements in the same period have the same number of electron shells. If the number of electrons present is higher, the attraction between the electrons and the nucleus is also high. In the beginning of the period, there is a less number of electrons present in the outermost orbital. Then the attraction from the nucleus is less. Therefore, the atom is large, and the atomic radius is also large. But when moving along a period, the number of protons in the nucleus is increased along with the number of electrons present in the atom. Therefore, the attraction force between electrons and the nucleus is high. It causes the size of the atom to shrink; then the atomic radius is reduced. Likewise, when moving along a period, the size of the atom is decreased gradually, so is the atomic radius.
What is the atomic radius?
What is Atomic Radius. Atomic radius is the distance from the nucleus of an atom to its boundary of the electron cloud. In other words, it is the distance from the nucleus to the farthest electron that belongs to that atom. Atomic radius can be defined only for isolated and neutral atoms.
Why does the ionic radius increase as we move down a group?
This is because a new electron shell is added per each period when we go downward a group.

Overview
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).
Trends
Ions may be larger or smaller than the neutral atom, depending on the ion's electric charge. When an atom loses an electron to form a cation, the other electrons are more attracted to the nucleus, and the radius of the ion gets smaller. Similarly, when an electron is added to an atom, forming an anion, the added electron increases the size of the electron cloud by interelectronic repul…
Determination
The distance between two ions in an ionic crystal can be determined by X-ray crystallography, which gives the lengths of the sides of the unit cell of a crystal. For example, the length of each edge of the unit cell of sodium chloride is found to be 564.02 pm. Each edge of the unit cell of sodium chloride may be considered to have the atoms arranged as Na ∙∙∙Cl ∙∙∙Na , so the edge is twice t…
Non-spherical ions
The concept of ionic radii is based on the assumption of a spherical ion shape. However, from a group-theoretical point of view the assumption is only justified for ions that reside on high-symmetry crystal lattice sites like Na and Cl in halite or Zn and S in sphalerite. A clear distinction can be made, when the point symmetry group of the respective lattice site is considered, which are the cubic groups Oh and Td in NaCl and ZnS. For ions on lower-symmetry sites significant deviation…
See also
• Atomic orbital
• Atomic radii of the elements
• Born equation
• Covalent radius
• Electride
External links
• Aqueous Simple Electrolytes Solutions, H. L. Friedman, Felix Franks