How many orbitals are in each sublevel?
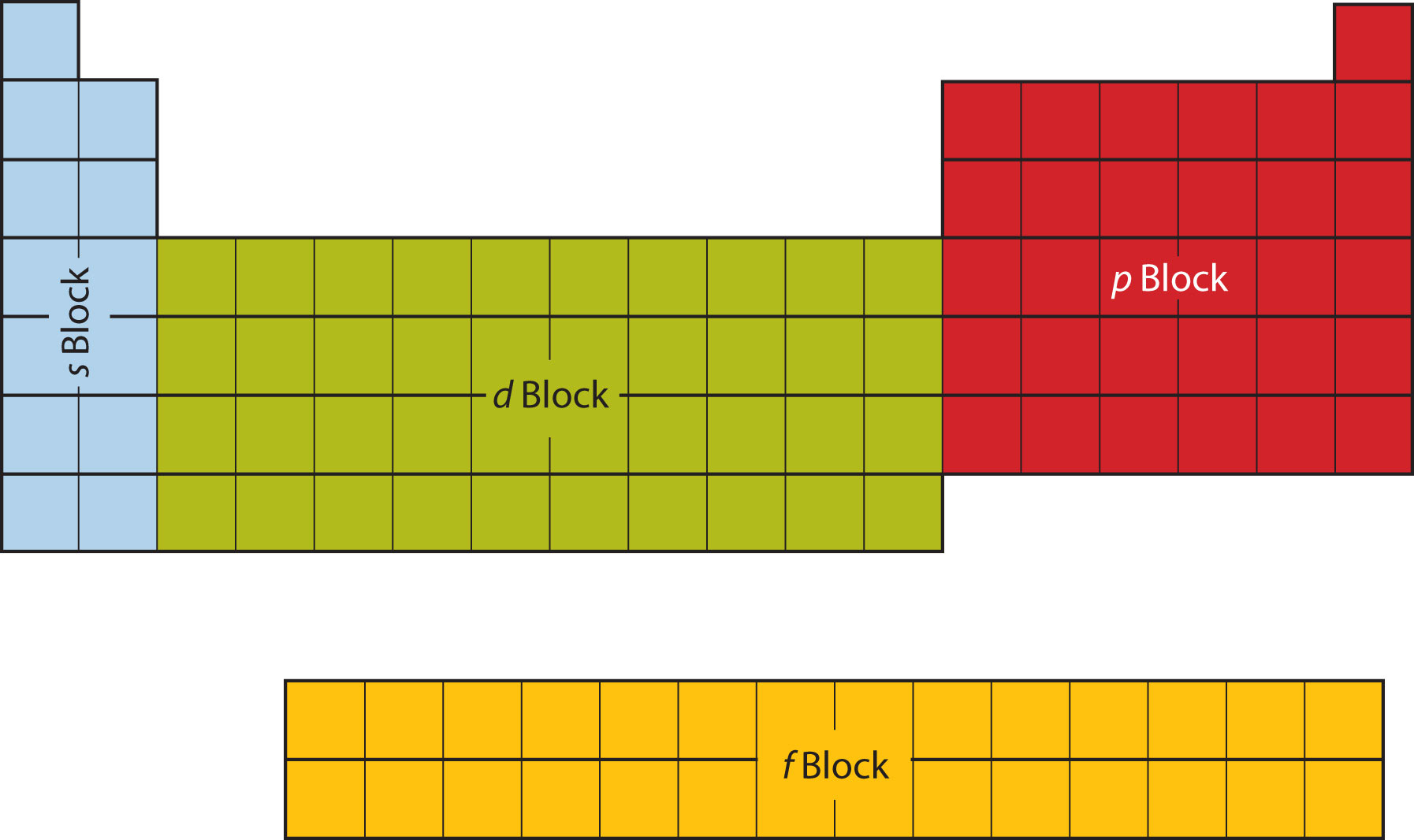
Each sublevel has differing numbers of orbitals. Sublevels are designated with lower-case letters. Sublevel s contains one orbital, p contains three, d has five, f has seven, g has nine, h has 11 and i has 13. The periodic table of elements contains seven rows; therefore, elements can have up to seven energy levels, depending on how many ...
What is the difference between an orbital and a sublevel?
What is the difference between orbitals and sublevels? The sublevels contain orbitals. Orbitals are spaces that have a high probability of containing an electron. In other words, an orbital is an area where the electrons live. There can be two electronsin one orbital maximum. The s sublevel has just one orbital, so can contain 2 electrons max.
What is a D shape?
They also struggled to create certain geometric shapes like seamless cones and domes. The researchers also found that the pasta best held its 3-D shape when cooked not much longer than 7 minutes. “In other words, the pasta can never not be al dente ...
What is the shape of the d orbital?
The d Orbitals
- The magnetic orbital quantum number for d orbitals is denoted as (-2, -1, 0, 1, 2). ...
- The d orbitals are given the designations d xy, d yz, d xz, d x2 - y2 and d z2.
- The shapes of the first four d orbitals are similar to one another while being different from the dz2 orbital.
- The energy of all five d orbitals is the same.
What are d orbitals shaped like?
The d orbital is a clover shape because the electron is pushed out four times during the rotation when an opposite spin proton aligns gluons with three spin-aligned protons.
What is the shape of d orbital answer?
Hence, we can say d-orbitals have double dumbbell-shaped.
What is the shape of the f sublevel?
tetrahedral structureThe f orbital has 15 protons to complete a fifth level of a tetrahedral structure. The f orbital is more complex, but follows the same rules based on proton alignment as the p and d orbitals. When completely full it is similar to the d orbital, but cut in half (eight lobes instead of four).
Is the d orbital a sphere?
There are four different kinds of orbitals, which are named s, p, d and f orbitals. They each have a different orbital shape. An s-orbital is spherical with the nucleus at its center. A p-orbital is dumbbell-shaped and four out of five d-orbitals are cloverleaf shaped.
How many orbitals are there in the d sublevel?
5 orbitalsThe d sublevel has 5 orbitals, so can contain 10 electrons max.
What is the shape of SP and d orbitals?
The atomic orbitals are of different shapes, where the s orbital has a spherical shape, the p orbital has a dumbbell shape, and four of the five d orbitals have a cloverleaf shape.
What does the S sublevel look like?
The s-sublevel is made up of a singular orbital holding a maximum of 2 electrons. It is a spherical shape. The 1s is the closest to the nucleus and is smaller that the 2s, which is smaller than the 3s and so on. The p-sublevel is made up of a 3 identical dumbbell like orbitals.
How many electrons are in d orbital?
10 electronsThis means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.
What does the D in d orbital mean?
The orbital names s, p, d, and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. These line groups are called sharp, principal, diffuse, and fundamental.
What elements have complete d orbitals?
This can be attributed to the relative stability of the completely filled d orbital. Zinc, Mercury, Cadmium, and Copernicium exhibit completely filled orbitals in their ground states and in their general oxidation states as well.
How are DXY and dx2 y2 orbitals related and draw shapes?
1 Answer. The dxy orbital is exactly same as dx2-y2 orbital and only difference is that its lobes are at an angle of 45° to the lobes of dx2-y2 Orbital.