
What is nitrate ion molecular shape?
Uses of NO3
- The nitrate (NO3) can be used as an organic or inorganic ester or salt of nitric acid, containing the (NO3-) ion.
- Of all salts, nitrates are the most soluble in water and play a significant role in the nitrogen cycle and nitrate pollution as well.
- The inorganic nitrates are formed by the bacteria and are essential components of agricultural soil.
What is the geometry of the nitrate ion?
There is one central atom in nitrate which is surrounded by 3 identically-bonded oxygen atoms that lie at the triangle corners and a similar one-dimensional plane. In essence, nitrate has 3 electron domains with zero lone pairs. Thus, NO3- molecular geometry is trigonal planar and is slightly bent. The bond angle is 120°.
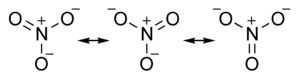
How many resonance structures does the nitrate ion No31- have?
There is no three resonance structures existing simultaneously and those resonance structures are not rotating from one structure to another. What actually happening is, there is only one real structure for nitrate ion (resonance hybrid). To draw that, we use above three resonance structures.
What is the ion of a nitrate?
The ion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1.
What is the structure of a nitrate ion?
In nitrate, there is one central atom which is surrounded by three identically-bonded oxygen atoms which lie at the corners of a triangle and at the same one-dimensional plane. In essence, nitrate has 3 electron domains and no lone pairs.
What type of structure is nitrate?
Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. Principal species present at pH 7.3. It is a nitrogen oxoanion, a member of reactive nitrogen species and a monovalent inorganic anion. It is a conjugate base of a nitric acid.
How do you draw a nitrate ion?
0:015:52How To Draw The Lewis Structure of NO3- (Nitrate Ion) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo let's begin one of the first things that we need to do in order to draw the lewis structure ofMoreSo let's begin one of the first things that we need to do in order to draw the lewis structure of any polyatomic ion is to count the number of valence electrons of each element in that ion.
Is nitrate ionic or covalent?
ionicSodium nitrate is an example of an ionic substance that contains such a group. Show activity on this post. The answer your teacher seems to have been looking for was to specify that the the sodium-nitrate bond is ionic and nitrate's nitrogen-oxygen bond is covalent.
Is no3 trigonal planar?
In essence, nitrate has 3 electron domains with zero lone pairs. Thus, NO3- molecular geometry is trigonal planar and is slightly bent.
Is no3 tetrahedral?
0:001:49NO3- Molecular Geometry / Shape and Bond Angles - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo when we look at the molecular geometry for the no3 minus molecule there's two ways we can do thisMoreSo when we look at the molecular geometry for the no3 minus molecule there's two ways we can do this the first way is to look at the valence shell electron pair repulsion theory.
Is no2 a structure?
Nitrogen Dioxide (NO2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm3.
Is nitrate a metal?
nitrate, chemical compound containing the nitrate (NO3) radical. Nitrates are salts or esters of nitric acid, HNO3, formed by replacing the hydrogen with a metal (e.g., sodium or potassium) or a radical (e.g., ammonium or ethyl).
How many lone pairs does an oxygen atom have?
Start to mark those nine valence electrons pairs on outside atoms (oxygen atoms) as lone pairs. One oxygen atom will take three lone pairs following the octal rule (oxygen and nitrogen atoms cannot keep more than eight electrons in their valence shells). All nine valence electrons pairs (9) are spent when lone pairs are marked on oxygen atoms.
How many atoms are in a nitrate ion?
There are one nitrogen atom and three oxygen atoms in the nitrate ion. Also there is a -1 charge on the nitrate ion.
Which atom has the most chance of being the center atom?
To be the center atom, ability of having greater valance is important. Nitrogen can show valence,5. But, oxygen's maximum valence is 2. Therefore nitrogen has the more chance to be the center atom (See the figure). So, now we can build a sketch of NO 3- ion.
Does nitrogen have electrons?
No electrons pairs exist on nitrogen atom. But, on nitrogen atom, there is a +1 charge. Around the nitrogen atom, there are two single bonds and double bond.
Is there an ine valence electron pair for nitrogen?
Therefore, there is no ine valence electrons pairs to mark on nitrogen atom.
Is there a double bond between nitrogen and oxygen?
Now there is a double bond between nitrogen and one oxygen atom. There are also two single bonds (N-O) with nitrogen atom and other oxygen atoms.
What is the Lewis dot structure?
Hint : The lewis dot structure includes marking the valence electrons of every element included in the compound with the electrons used in the covalent as well as ionic bonds. For simplicity, we can use different symbols to show electrons of different elements.
How is nitrate ion formed?
Nitrate ion is formed from Hydrogen Nitrate, when the hydrogen atom leaves the compound as a positive ion , leaving its electron behind, which makes the charge on nitrate ion negative.
Why does nitrate have a negative charge?
The fact to remember here is that the nitrate ion is formed from Hydrogen Nitrate and hence, will always have a negative charge due to higher electronegativity. The negative or positive should always be included in the total counting of electrons of the compound. Here, the factors like bond length and bond angle can be ignored safely. Hence, the structure is not considered wrong if the bond angle is wrong. The important thing is to show the correct sharing of electrons.
How many valence electrons are in nitrogen?
We know there are five valence electrons in nitrogen and six valence electrons in oxygen.
What does the plus sign mean in chemistry?
Here, plus sign shows electrons of nitrogen, dot shows electrons of oxygen, and minus sign shows electron of hydrogen.
What is the nitrate in meat?
Cured meats. Nitrite consumption is primarily determined by the amount of processed meats eaten, and the concentration of nitrates in these meats. Although nitrites are the nitrogen compound chiefly used in meat curing, nitrates are used as well. Nitrates lead to the formation of nitrosamines.
What is the result of lightning strikes?
Lightning strikes in earth's nitrogen-oxygen rich atmosphere produce a mixture of oxides of nitrogen which form nitrous ions and nitrate ions which are washed from the atmosphere by rain or in occult deposition . Nitrates are produced industrially from nitric acid.
How are nitrates produced?
Nitrates are produced by a number of species of nitrifying bacteria in the natural environment using ammonia or urea as a source of nitrogen . Nitrate compounds for gunpowder were historically produced, in the absence of mineral nitrate sources, by means of various fermentation processes using urine and dung.
What are salts containing nitrates called?
Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate .
Why are nitrates used in agriculture?
Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability. The main nitrate fertilizers are ammonium, sodium, potassium, calcium, and magnesium salts. Several million kilograms are produced annually for this purpose.
What are the symptoms of nitrate poisoning in domestic animals?
Symptoms of nitrate poisoning in domestic animals include increased heart rate and respiration ; in advanced cases blood and tissue may turn a blue or brown color. Feed can be tested for nitrate; treatment consists of supplementing or substituting existing supplies with lower nitrate material.
What is sodium nitrate used for?
Sodium nitrate is used to remove air bubbles from molten glass and some ceramics. Mixtures of the molten salt are used to harden some metals. Nitrate was also used as a film stock through nitrocellulose. Due to it's high combustibility, the studios swapped to acetate safety film in 1950.
What is a formal charge?
Somewhere, every atom has a formal charge on it. Formal charge plays an important role in Lewis dot structure.
How many valence electrons does each oxygen atom have?
Firstly, complete the octet of the terminal atoms. From the remaining 18 valence electrons, arrange them in such a way that each oxygen atom receives 6 valence electrons and form 3 lone pairs. 8. After noticing nitrogen, it has only 6 valence electrons.
How many valence electrons are needed to make a dot structure of nitrate?
5. Begin the framing dot structure of nitrate by making 3 single bonds between 3 atoms of oxygen and nitrogen. 6 valence electrons are used.
How many atoms are in NO3?
1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge.
What is the Lewis dot structure?
Below are some rules to frame any compound’s Lewis dot structure. 1. Follow the octet rule where an atom should complete its outermost shell by the total number of 8 electrons. (Exceptions are hydrogen and boron elements)
What is the F.C of oxygen?
F.C of oxygen making single bond with the nitrogen atom. F.C = 6 – 6 – (2/2) = -1 i.e. both the oxygen atoms making a single bond with nitrogen have a negative formal charge. To calculate the total charge of the nitrate ion, a pair of +ve and –ve formal charges get canceled and there is only one –ve formal charge left on the oxygen atom.
Why is nitrate important?
It is used as fertilizers (like ammonium, sodium, potassium) in agricultural farms for higher solubility and biodegradability. It also treats heart pains. Both nitrogen and oxygen are important to an ecosystem that includes flora and fauna.
How many electrons does a nitrogen atom have?
You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with nitrogen atom. With that, total electrons around nitrogen atom is going to be ten. It is going to break octal rule because nitrogen atom cannot keep more than eight electrons in its last shell.
How many lone pairs are there in the last shell of an oxygen atom?
That oxygen atom is connected to the nitrogen atom by a double bond has two lone pairs in its last shell. Also, there is no charge in that oxygen atom. On nitrogen atom, there are no lone pair. But there is +1 charge on nitrogen atom.
How many lone pairs of electrons are in each of the three resonance structures of NO3?
each of the three resonance structures of no3– has how many lone pairs of electrons? There are eight lone pairs of electrons in each of the three resonance structures of NO 3- ion. These eight lone pairs are provides by three oxygen atoms. There are no lone pairs on nitrogen atom.
How many resonance structures are there?
There is no three resonance structures existing simultaneously and those resonance structures are not rotating from one structure to another. What actually happening is, there is only one real structure for nitrate ion (resonance hybrid). To draw that, we use above three resonance structures.
How many oxygen atoms are in a nitraate ion?
In all stable resonance structures of nitraate ion, two oxygen atoms have charges.
Is nitrate a Lewis structure?
Each of stable resonance structure of nitrate ion is a lewis structure.
