| Isotopes | Isotopes | Half-life (years) | Effective Dating Range (years) |
| Dating Sample | Key Fission Product | ||
| Lutetium-176 | Hafnium-176 | 37.8 billion | early Earth |
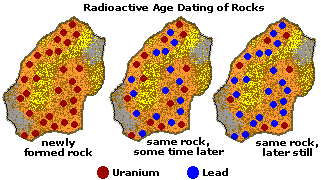
| Uranium-238 | Lead-206 | 4. 468 billion | 10 million to origin of Earth |
| Uranium-235 | Lead-207 | 704 m illion | 10 million to origin of Earth |
Which element can be used in radioactive dating?
Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. Likewise, are all elements useful for radioactive dating? Many different radioactive isotopes and techniques are used for dating.
What are four uses of radioactive isotopes?
Radioactive isotopes have a variety of applications such as medical imaging, treating of cancer, tracing steps of chemical reactions, establishing the ages of objects, and generating electricity. Radioactive isotopes are effective tracers because their radioactivity is easy to detect. A tracer is a substance that can be used to follow a ...
What is the role of isotopes in radiometric dating?
What is the role of isotopes in radiometric dating? A radioactive isotope decays into its stable daughter product at a constant rate. The time it takes for half of the isotope in a sample to decay is known as the half-life of the isotope.
What are the beneficial uses of radioactive isotopes?
Radioactive isotopes have many useful applications. In medicine, for example, cobalt-60 is extensively employed as a radiation source to arrest the development of cancer. Other radioactive isotopes are used as tracers for diagnostic purposes as well as in research on metabolic processes.
What is radioactive dating?
How many naturally occurring isotopes are there?
How does uranium decay?
What is the Uranium-Lead system?
What is 147Sm Nd used for?
How to date a rock?
How to see fission tracks?
See 4 more
About this website

What substances are used for radioactive dating?
Major radioactive elements used for radiometric dating. Zircon, Uraninite. Muscovite, Biotite, volcanic rocks. Muscovite, Biotite, Metamorphic or Igneous rocks.
Which elements isotopes are used for dating samples?
The most widely used radioactive cosmogenic isotope is carbon of mass 14 (14C), which provides a method of dating events that have occurred over roughly the past 60,000 years.
What isotopes can be used in radiometric dating and why?
Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years.
What are 4 types of radiometric dating?
Methods used for radiometric dating are uranium-lead dating, potassium-argon dating, rubidium-strontium dating, and radiocarbon dating. In uranium-lead dating, uranium -238 decays to lead-206 and uranium-235 decays to lead-207.
What is isotopic dating used for?
Summary. Isotopic dating is the measurement of time using the decay of radioactive isotopes and accumulation of decay products at a known rate. With isotopic chronometers, we determine the time of the processes that fractionate parent and daughter elements.
What type of isotopes are most commonly used to date geologic materials?
One of the isotope pairs commonly used to date rocks is the decay of 40K to 40Ar (potassium-40 to argon-40). K is a radioactive isotope of potassium that is present in very small amounts in all minerals that contain potassium.
What type of element is being used for absolute dating?
Absolute dating methods measure the physical properties of an object itself and use these measurements to calculate its age. One of the most useful absolute dating methods for archaeologists is called radiocarbon dating. It works by measuring carbon isotopes, which are versions of the element carbon.
What element is used in geological dating?
To establish the age of a rock or a fossil, researchers use some type of clock to determine the date it was formed. Geologists commonly use radiometric dating methods, based on the natural radioactive decay of certain elements such as potassium and carbon, as reliable clocks to date ancient events.
Radiometric Dating: Methods, Uses & the Significance of Half-Life
Radiometric Dating. The aging process in human beings is easy to see. As we age, our hair turns gray, our skin wrinkles and our gait slows. However, rocks and other objects in nature do not give ...
Radiometric Dating: Definition, How Does it Work, Uses & Examples
Radiometric dating is a means of determining the age of very old objects, including the Earth itself. Radiometric dating depends on the decay of isotopes, which are different forms of the same element that include the same number of protons but different numbers of neutrons in their atoms.
Radiometric Age Dating - Geology (U.S. National Park Service)
Thermal ionization mass spectrometer used in radiometric dating. Radiometric dating calculates an age in years for geologic materials by measuring the presence of a short-life radioactive element, e.g., carbon-14, or a long-life radioactive element plus its decay product, e.g., potassium-14/argon-40.
Radiometric dating - Understanding Evolution
Geologists use radiometric dating to estimate how long ago rocks formed, and to infer the ages of fossils contained within those rocks. Radioactive elements decay The universe is full of naturally occurring radioactive elements. Radioactive atoms are inherently unstable; over time, radioactive "parent atoms" decay into stable "daughter atoms." When molten rock cools, forming what
Radioactive Dating | Encyclopedia.com
Radioactive Dating. In the nineteenth century, prominent scientists such as Charles Lyell, Charles Darwin, Sir William Thomson (Lord Kelvin), and Thomas Huxley, were in continual debate about the age of the earth.The discovery of the radioactive properties of uranium in 1896 by Henri Becquerel subsequently revolutionized the way scientists measured the age of artifacts and supported the theory ...
Why are radioactive isotopes useful?
Generally, however, they are useful because either we can detect their radioactivity or we can use the energy they release. Radioactive isotopes are effective tracers because their radioactivity is easy to detect. A tracer is a substance that can be used to follow the pathway ...
What is the purpose of carbon-14?
One isotope, carbon-14, is particularly useful in determining the age of once-living artifacts. A tiny amount of carbon-14 is produced naturally in the upper reaches of the atmosphere, and living things incorporate some of it into their tissues, building up to a constant, albeit very low, level.
What is the half life of iodine 131?
Iodine-131 has a half-life of only 8 d , so the potential for damage due to exposure is minimal. Technetium-99 can also be used to test thyroid function. Bones, the heart, the brain, the liver, the lungs, and many other organs can be imaged in similar ways by using the appropriate radioactive isotope.
What is the purpose of irradiating food?
The radiation emitted by some radioactive substances can be used to kill microorganisms on a variety of foodstuffs, extending the shelf life of these products. Produce such as tomatoes, mushrooms, sprouts, and berries are irradiated with the emissions from cobalt-60 or cesium-137.
How can tracer be used in chemistry?
Tracers can also be used to follow the steps of a complex chemical reaction. After incorporating radioactive atoms into reactant molecules, scientists can track where the atoms go by following their radioactivity. One excellent example of this is the use of carbon-14 to determine the steps involved in photosynthesis in plants.
Which gland absorbs the most iodine?
The thyroid gland absorbs most of the iodine, allowing it to be imaged for diagnostic purposes or preferentially irradiated for treatment purposes.
What is the disintegration rate of carbon 14?
The current disintegration rate for carbon-14 is 14.0 Bq. A sample of burnt wood discovered in an archaeological excavation is found to have a carbon-14 disintegration rate of 3.5 Bq. If the half-life of carbon-14 is 5,730 y, approximately how old is the wood sample?
Why can’t we use isotopic dating techniques with sedimentary rocks?
An important assumption that we have to be able to make when using isotopic dating is that when the rock formed none of the daughter isotope was present (e.g., 40 Ar in the case of the K-Ar method). A clastic sedimentary rock is made up of older rock and mineral fragments, and when the rock forms it is almost certain that all of the fragments already have daughter isotopes in them. Furthermore, in almost all cases, the fragments have come from a range of source rocks that all formed at different times. If we dated a number of individual grains in the sedimentary rock, we would likely get a range of different dates, all older than the age of the rock. It might be possible to date some chemical sedimentary rocks isotopically, but there are no useful isotopes that can be used on old chemical sedimentary rocks. Radiocarbon dating can be used on sediments or sedimentary rocks that contain carbon, but it cannot be used on materials older than about 60 ka.
What is radiocarbon dating?
Radiocarbon dating (using 14 C ) can be applied to many geological materials, including sediments and sedimentary rocks, but the materials in question must be younger than 60 ka. Fragments of wood incorporated into young sediments are good candidates for carbon dating, and this technique has been used widely in studies involving late Pleistocene glaciers and glacial sediments. An example is shown in Figure 8.16; radiocarbon dates from wood fragments in glacial sediments have been used to estimate the time of the last glacial advance along the Strait of Georgia.
What is the decay of 40 K?
It is also based on the premise that when the atoms of an element decay within a mineral or a rock, they stay there and don’t escape to the surrounding rock, water, or air. One of the isotope pairs widely used in geology is the decay of 40 K to 40 Ar (potassium-40 to argon-40). 40 K is a radioactive isotope of potassium ...
What type of rock is used to date K-Ar?
In order to use the K-Ar dating technique, we need to have an igneous or metamorphic rock that includes a potassium-bearing mineral. One good example is granite, which normally has some potassium feldspar (Figure 8.15). Feldspar does not have any argon in it when it forms.
What is the proportion of 40 K remaining in feldspar?
Assume that a feldspar crystal from the granite shown in Figure 8.15 was analyzed for 40 K and 40 Ar. The proportion of 40 K remaining is 0.91. Using the decay curve shown on this graph, estimate the age of the rock.
Why do fossils only provide relative ages?
Originally fossils only provided us with relative ages because, although early paleontologists understood biological succession, they did not know the absolute ages of the different organisms. It was only in the early part of the 20th century, when isotopic dating methods were first applied, that it became possible to discover the absolute ages ...
Can sedimentary rocks be dated isotopically?
If we dated a number of individual grains in the sedimentary rock, we would likely get a range of different dates, all older than the age of the rock. It might be possible to date some chemical sedimentary rocks isotopically, but there are no useful isotopes that can be used on old chemical sedimentary rocks.
What is radioactive dating?
Radioactive dating is a method of dating rocks and minerals using radioactive isotopes. This method is useful for igneous and metamorphic rocks, which cannot be dated by the stratigraphic correlation method used for sedimentary rocks. Over 300 naturally-occurring isotopes are known.
How many naturally occurring isotopes are there?
Over 300 naturally-occurring isotopes are known. Some do not change with time and form stable isotopes (i.e. those that form during chemical reactions without breaking down). The unstable or more commonly known radioactive isotopes break down by radioactive decay into other isotopes.
How does uranium decay?
Several minerals incorporate tiny amounts of uranium into their structure when they crystallise. The radioactive decay from the uranium releases energy and particles (this strips away electrons leading to disorder in the mineral structure). The travel of these particles through the mineral leaves scars of damage about one thousandth of a millimetre in length. These 'fission tracks' are formed by the spontaneous fission of 238U and are only preserved within insulating materials where the free movement of electrons is restricted. Because the radioactive decay occurs at a known rate, the density of fission tracks for the amount of uranium within a mineral grain can be used to determine its age.
What is the Uranium-Lead system?
Uranium-Lead (U-Pb) system. This system is highly favoured for accurate dating of igneous and metamorphic rocks, through many different techniques. It was used by the beginning of the 1900s, but took until the early 1950s to produce accurate ages of rocks.
What is 147Sm Nd used for?
It is used for very old to very young rocks. Samarium-Neodymium (Sm-Nd) The decay of 147Sm to 143Nd for dating rocks began in the mid-1970s and was widespread by the early 1980s. It is useful for dating very old igneous and metamorphic rocks and also meteorites and other cosmic fragments.
How to date a rock?
Either a whole rock or a single mineral grain can be dated. Some techniques place the sample in a nuclear reactor first to excite the isotopes present, then measure these isotopes using a mass spectrometer (such as in the argon-argon scheme). Others place mineral grains under a special microscope, firing a laser beam at the grains which ionises the mineral and releases the isotopes. The isotopes are then measured within the same machine by an attached mass spectrometer (an example of this is SIMS analysis).
How to see fission tracks?
To see the fission tracks, the mineral surface is polished, etched with acids, and examined with an electron microscope. An effective way to measure the uranium concentration is to irradiate the sample in a nuclear reactor and produce comparative artificial tracks by the induced fission of 235U.